Starton’s proprietary continuous delivery technology can increase efficacy of approved drugs, make them more tolerable, and expand their potential use. We are advancing the following candidates in our continuous delivery platform:
STAR-LLD: Transdermal and continuous subcutaneous formulations of lenalidomide for chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and other cancers. STAR-LLD has the potential to become the first immunomodulatory drug (IMiD) in CLL, and a best-in-class IMiD in MM.
Our platform technology reduces the area under the curve (AUC) and maximum plasma concentration (Cmax) of a drug to improve tolerability and avoid periods of subtherapeutic dosing due to a short elimination half-life (T1/2).
Our company is led by a world-class team comprised of experienced industry professionals and clinical opinion leaders. We strengthen our capabilities through collaborations with industry-leading firms. We are committed to developing bold solutions to address unmet medical needs, moving together with quality and speed to deliver on our commitment to patients.
CLL and MM are the most common blood cancers in the US with 21,250 and 34,920 new diagnoses expected in 2021, respectively. Both CLL and MM are rarely curable. Although current treatments extend survival for an average of three to 10 years, deteriorating quality of life remains a challenge due to drug-related side-effects
If dose-related side effects can be reduced and people can tolerate maintenance therapy for decades to help stay in remission, these cancers, that were previously a death sentence, would become more akin to a chronic disease with a long lifespan and healthy quality of life.
STAR-LLD uses novel treatment modalities of continuous delivery of low-dose lenalidomide. The use of continuous delivery of lenalidomide allows for lower peaks and higher troughs which provide therapeutic blood levels for the entirety of the dosing interval whereas once-daily oral dosing is associated with sub-therapeutic blood levels of lenalidomide during each days’ dosing. Importantly, the total daily exposure is 55-70% lower than once-daily oral administration with a resultant AUC which is similarly lower than oral therapy. The expected result of this “flattening of the curve” of the blood levels is better tolerability through lower exposure and improved efficacy through continuous maintenance of the minimum immunomodulatory concentration of the treatment interval.
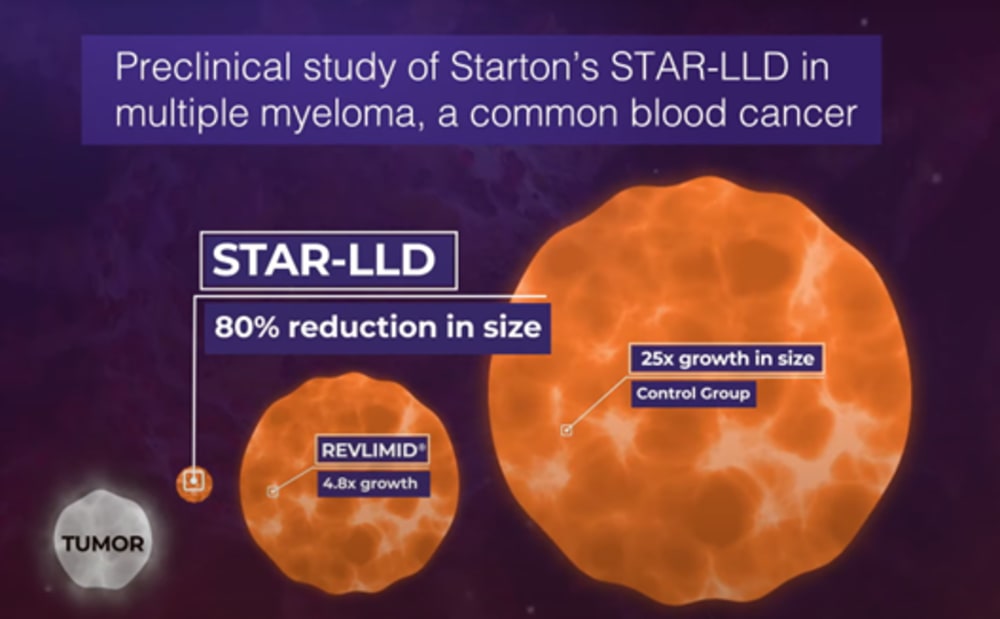
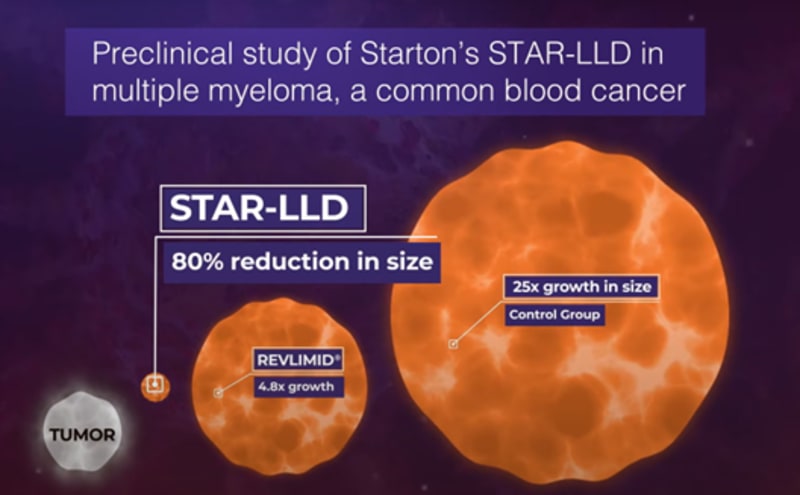
These preliminary data support the premise that the administration of a continuous, lower dose of an anti-tumor drug results in better tumor response and no additional toxicity, Starton is advancing two continuous delivery formulations towards the clinic: subcutaneous (SC) and transdermal delivery system (TDS).
A Phase 1 clinical study to assess pharmacokinetics versus the oral formulation was completed successfully, meeting all primary and secondary endpoints. A Phase 1b study is currently ongoing evaluating the safety, efficacy, and PK of continuous subcutaneous administration of low-dose STAR-LLD in patients with multiple myeloma. Starton also intends to conduct a Phase 1/2 study in chronic lymphocytic leukemia (CLL). Starton has confirmed with FDA that STAR-LLD will follow a 505(b)2 regulatory path for pre-clinical development of STAR-LLD and intends to conduct a full clinical program in its target indications.
Video
-
Awards
-
 2024 Top 100 Entries
2024 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Rod Hartwig
- Type of entry:individual