Hydrogen combustion is eco-friendly: its combustion returns water to the ecosystem. Water electrolysis – a green hydrogen concept – coupled with proton permeable membrane technology is one way to generate hydrogen. The main challenge with this technique is the high electric power consumption required to split water. This has made it impossible to deploy sustainable H2 energy for base resiliency and expediency forces. However, water, which is the sole hydrogen source in water electrolysis is becoming increasingly scarce. This is coupled with increasing electricity tariffs. Solution-wise, the next generation of sustainability will explore more of circular economics – the duality of reuse and regeneration of materials while eliminating waste/pollution. In this case, successfully coupling wastewater treatment (for reuse) to hydrogen generation (regeneration).
On the other hand, with increasing legal battles and settlements over decades-long PFAS contamination and no economical and easily scalable solution for total PFAS eradication (See Figure 1), the wider population (PFAS is a world-wide contaminant) are reeling from consequences of ubiquitous PFAS presence in our environment and subsequent contaminations in water – even aquifers, and foods such as different cancer forms, developmental issues in children, teratogenicity, thyroid issues, to mention a few.
A cost-effective and efficient secular economic solution that couples efficient electro-oxidation of wastewater at the anode to proton reduction and hydrogen generation at the cathode is proposed. PFAS contaminated water (and even domestic wastewater domestic wastewater is mainly organic and inorganic waste substances such as skin- care products, detergents, and pharmaceuticals to mention a few) are usually in hydrogen-rich matrices even though PFAS, as a group of compounds, are Fluorine dominated.
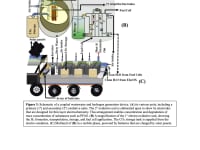
An innovative graphite thermal treatment protocol resulted in electron transfer properties on the graphite that are better than those of Platinum (Pt), Gold (Au), and Palladium (Pd), which are the industry standards for electro-catalysis. This electro-oxidation involves electrons (at a current density of 5 mA/cm2) as the sole abstracting and degrading/disinfecting agent in wastewater (See Figure 2). These abstracted protons (H+) from the backbones of organic and inorganic wastes, are reduced at the cathode. These released hydrogen gases can be collected, bottled, and used for any energy use of importance (See Figure 3).
A descriptive energy analysis (DEA) and Techno-economic analysis (TEA) show that the electrical energy consumed to reduce H2 from Methylene Blue is four times less than the actual energy required to break the bonds. In practical terms, the energy consumed is ten times less than the most efficient electrolyzers to generate similar quantities of H2. The current efficiency analysis reveals that 80% of the applied electrical energy went into abstracting and reducing Hydrogen from MB, while the remaining 20% generated hydrogen from water splitting (corrosion percentage was calculated from electrode weight losses, is 0.00010%).
Commercially, this device would go for large scale PFAS clean-up when coupled to a concentrating ion-exchange or reverse-osmosis set-up. The oil & gas, aviation (military and civilian), battery, cosmetics, textile, paper mill/pulp industries are some potential clients.
Video
Like this entry?
-
About the Entrant
- Name:Okechukwu Nwamba
- Type of entry:teamTeam members:
- Okechukwu Nwamba
- David Aston
- Jean'ne Shreeve
- Software used for this entry:PowerPoint