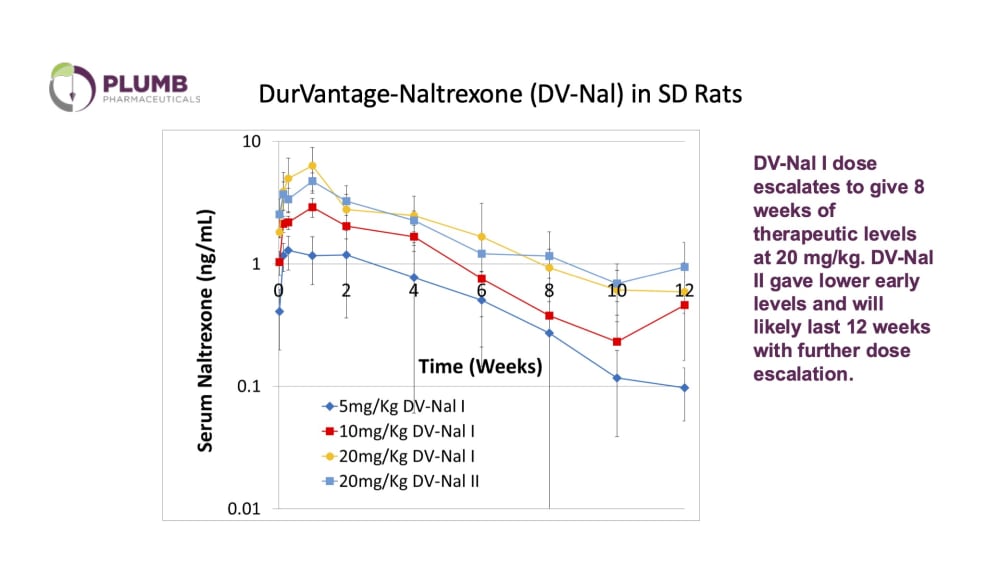
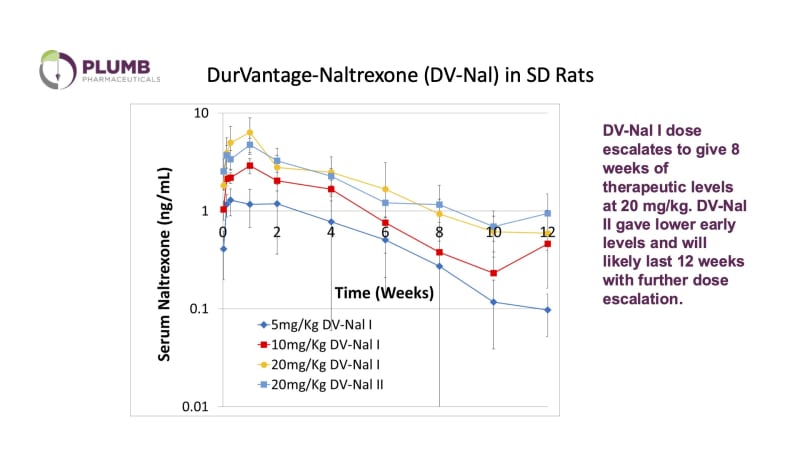
Opioid addiction is a major public health crisis that is becoming worse, 2.4 million Americans have been diagnosed with opioid dependence, and overdoses have increased, creating and economic burden of more than $78 billion/yr. Drug overdose deaths in 2021 were highest in people 35-44 years of age (62 deaths/100,000 in 2021) according to the CDC, when individuals are likely to have dependent children, and are likely members of the work force. Overdose deaths have dropped somewhat in recent years, but budget cuts in programs like the elimination of the federal support for naloxone, may reverse those gains. Medications, including methadone, buprenorphine and naltrexone are the gold standard for treatment to help curb cravings and, in the case of naltrexone, block opioid receptors. Naltrexone, an opioid antagonist, is useful in the treatment of Opioid Use Disorder (OUD), Alcohol Use Disorder (AUD) and, in combination with the antidepressant bupropion, in the treatment of Methamphetamine use. Naltrexone is a complete opioid antagonist and has no reward function to drive medication compliance. Compliance is improved by depot or extended-release medication compared to daily oral administration. The current approved extended-release naltrexone product, Vivitrol, lasts only 3-4 weeks and can cause significant pain and inflammation at the site of injection. Plumb’s solution of Durvantage-Naltrexone (DV-Nal) will require dosing every 3 months reducing the opportunity for relapse between doses and Fewer Clinic Visits. Our drug delivery platform utilizes lipid nanoparticles, also known as liposomes, which have been used in previously marketed drug formulations.
Our company has patented novel methods of forming and loading the liposomes that encapsulates higher concentrations of material and produces slower release of the drug. The resulting liposome has a high concentration of drug within the particle and then releases the drug very slowly after subcutaneous injection in animals. The finished, FDA-approved product will be injected subcutaneously by either a nurse in a physician’s office, or by a pharmacist. Plumb’s “customer” will be a pharma company expanding their IP inventory. Plumb will license DV-Nal to a pharma partner with patent risks or be acquired by the large pharmaceutical company. Vivitrol, the chief competitor to DV-Nal, has annual revenue over $300M and its patent protection will expire in 2029. Naltrexone implants, such as the O’Neil formulation or the magnesium stearate-based Prodetoxone, are available in other countries, but have never achieved market traction in the United States, chiefly because the minor surgical procedure necessary to apply them is not favored or available to the psychiatrists and other mental health professionals who treat OUD in the United States.
Like this entry?
-
About the Entrant
- Name:Lisa Krugner Higby
- Type of entry:individual
- Software used for this entry:No