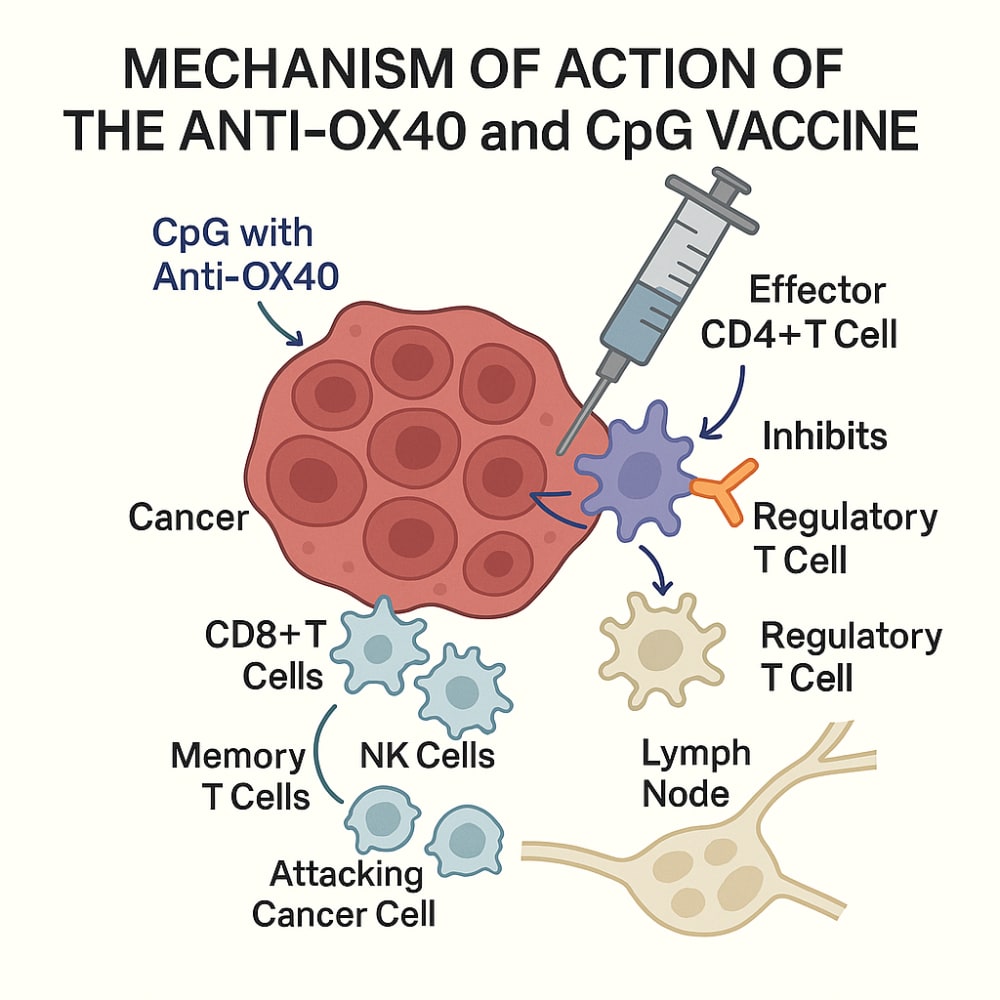
This project describes the development of a novel cancer immunotherapy vaccine designed to cure tumoral cancers in dogs. This vaccine is unique since the dog must have cancer for it to work. The vaccine employs a combination of an agonist anti-OX40 monoclonal antibody and a CpG oligodeoxynucleotide (ODN), delivered intratumorally. Its mechanism of action leverages the tumor microenvironment as the antigen source: the CpG stimulates upregulation of OX40 on effector CD4+ T cells while the anti-OX40 monoclonal antibody binds and dampens regulatory T cell (Treg) responses, effectively “releasing the brakes” on immune activation. This results in robust stimulation of cytotoxic CD8+ T cells and NK cells, driving local and systemic tumor destruction and formation of durable anti-tumor immune memory.
Innovation arises from three aspects:
- the vaccine is cancer-agnostic, requiring no prior identification of tumor antigens;
- it treats existing tumors and vaccinates against recurrence; and
- it is administered locally by general veterinarians rather than specialized oncology clinics. This stands in contrast to conventional approaches such as tumor-specific vaccines, CAR-T therapy, or checkpoint inhibitors, which are typically expensive, cancer-type specific, and logistically complex.
The production strategy relies on established biomanufacturing capabilities: the anti-OX40 monoclonal antibody was developed using canine OX40 protein to screen and optimize IgG-producing hybridomas, with the final clone candidates (7B7-3A10 and 3A11-8D7) selected for high affinity and manufacturability. The CpG ODN is synthesized by a cGMP-certified contractor. The vaccine components are mixed prior to injection into the tumor. Careful Uplift, the developer, will scale production under a USDA-approved Outline of Production, mitigating regulatory and process risk.
Feasibility is underpinned by preclinical canine trials demonstrating partial or complete tumor responses across multiple cancer types, including oral melanoma, mast cell tumors, mammary adenocarcinoma, sarcoma, and lymphoma. Three dogs achieved complete responses, with tumors shrinking by up to 99%. These encouraging outcomes provide evidence of broad applicability across tumor types. The regression in both high-grade and aggressive malignancies underscore the vaccine’s capacity to generate potent immune engagement regardless of tumor origin, offering a clear path for further clinical validation. Importantly, these results show the potential for durable remission and resistance to recurrence in a real-world veterinary setting, reinforcing the innovation’s transformative impact. The data also support scalability to larger canine populations and feasibility for international regulatory approval.
The marketability potential is significant: an estimated 4–6 million U.S. dogs are diagnosed with cancer annually. Current treatments prioritize remission rather than cure and are often prohibitively expensive or complex. By contrast, this vaccine offers a simpler, lower-cost, broadly applicable solution that can be administered by any veterinarian. The addressable market is forecast to exceed $1 billion annually, with an initial target price of approximately $1,500 per treatment course. Owners are likely to adopt this therapy, motivated by the opportunity to achieve lasting remission and protect their pets from recurrence.
If successful, this technology will transform veterinary oncology practice, improve canine survival outcomes, and create a commercially viable therapeutic platform potentially extensible to other species and ultimately human cancer treatment paradigms worldwide.
Like this entry?
-
About the Entrant
- Name:Ed Born
- Type of entry:individual
- Software used for this entry:No
- Patent status:pending