EXECUTIVE SUMMARY – TOURNIQUET COUPING DEVICE
The Tourniquet Coupling Device was designed from the perspective of patient comfort, patient safety, ease of use, disposability and low cost. All of these objectives have been met – thus dramatically enhancing the use of disposable latex and latex-free tourniquets.
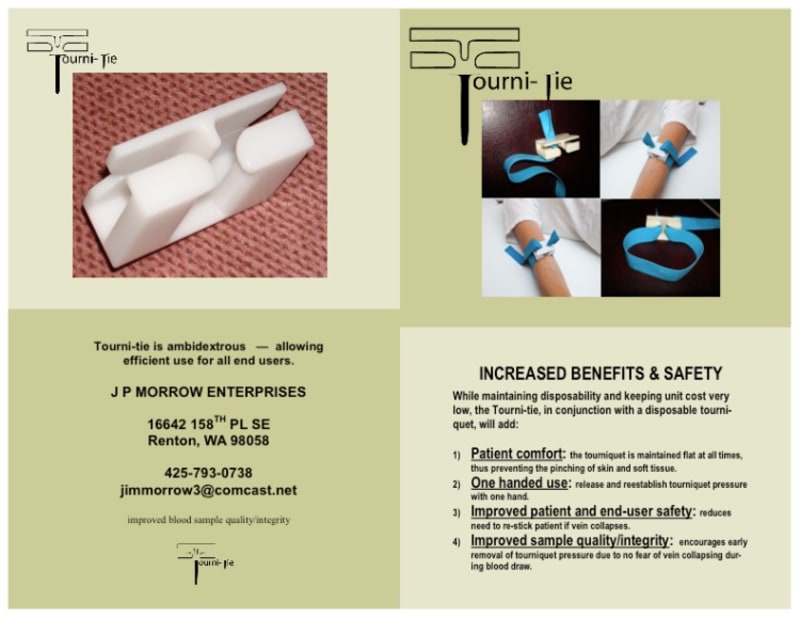
TECHNOLOGY: The device was designed to be a molded part using FDA approved material for noninvasive medical devices. The device is also ambidextrous, allowing equally efficient use by both right and left handed end users. Further, the device was designed with no moving parts and is of a size to allow for mass production, low unit cost, and disposability.
VALUE: The primary value of the device is patient satisfaction and safety, while enhancing the use of latex and latex-free tourniquets.
For most patients, the application of a standard disposable latex or latex-free tourniquet causes discomfort and/or pain for the balance of the blood draw. Further, according to recent studies on biochemistry and immunological tests, the acceptable period for tourniquet application time during phlebotomy is recommended to be 30-60 sec. Therefore, many times when numerous tubes are required for a patient blood sample, the tourniquet needs to be removed during the middle of the procedure to meet these procedural requirements. For many patients, this may cause the vein to collapse, thus not allowing the balance of the tubes to be drawn, and requiring the patient to be re-stuck, causing additional discomfort and pain for the patient, and increasing the risk of injury to the patient and end-user.
The Tourniquet Coupling Device eliminates these problems by first allowing the tourniquet to remain flat at all times during application with no pinching of the surrounding tissues. Second, upon application, one-hand operation allows for numerous applications of tourniquet pressure during the blood draw, eliminating the need to withdraw needle, reapply tourniquet and re-stick patient if the vein collapses after tourniquet pressure has been removed during the blood draw. Further, due to the device’s low cost and some procedural requirements for noninvasive medical devices, the device is disposable, minimizing the spread of disease. Also, the built-in quick release catch-design provides instant removal upon completion of the blood draw, further improving patient comfort and end-user safety.
1) Sample quality - allowing timely removal of tourniquet pressure.
2) Patient and end-user safety – no re-sticking of patient.
3) Patient comfort - the tourniquet is maintained in a flat position at all times.
Even though they are designed as a single use disposable product to ensure no cross contamination between patients, from a physical property standpoint, they can be reused many times if the market place deems that.
DISPOSABLE TOURNIQUET USAGE
MEDLINE USAGE/YR
Latex free: $1.6mil/yr
Latex $ .2mil/yr
Total = $1.8mil/yr
@$ .3 per unt = 6,000,000
@.25% usage w/unit cost @$ .5, gross revenues = $750,000
Just with Medline!
VA HOSPITAL USAGE – ALL STATES
Approximately 6,552,000 units used annually
Like this entry?
-
About the Entrant
- Name:Jim Morrow
- Type of entry:individual
- Software used for this entry:Alibre Design
- Patent status:pending