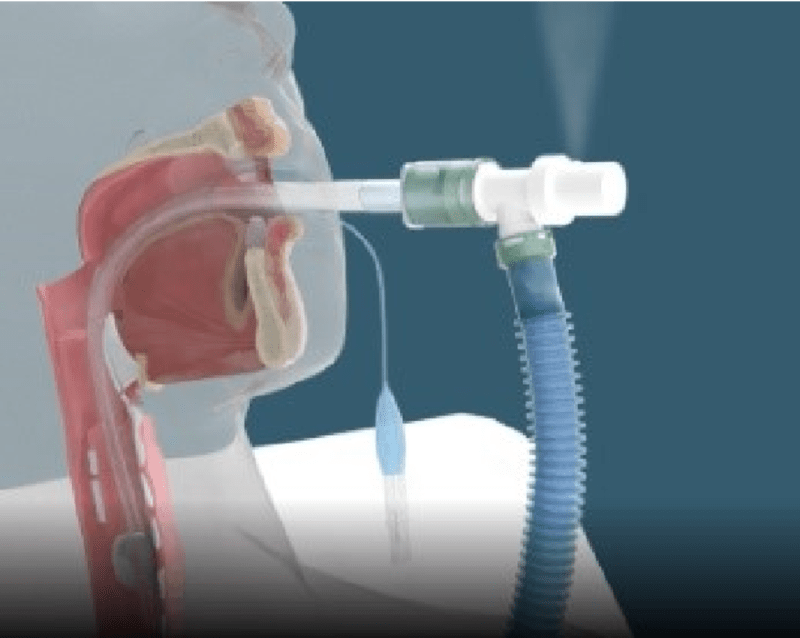
THE PROBLEM: Patients undergoing surgery may experience an accidental endotracheal tube disconnection from the anesthesia/ventilator breathing circuit. This dangerous accidental disconnect is known clinically as “pop-off”. When a “pop-off” disconnection occurs, the patient may suffer serious, permanent injury or death due to inadequate sedation and oxygenation.
OUR SOLUTION: Our patented POPSafe™ Endotracheal Tube (POPSafe™ ETT) is a medical device that eliminates deadly accidental anesthesia/ventilator “pop-off” disconnection events. Our POPSafe™ Endotracheal Tubes are the only products addressing the failure root cause of accidental anesthesia/ventilator breathing circuit disconnections. Our POPSafe™ Endotracheal Tubes will be the first and only FDA 510(k) cleared endotracheal tubes which include our patented ventilator elbow connector and patented over-pressure relief safety valve. POPSafe™ ETT is a next-generation endotracheal tube device intended to prevent dangerous, accidental anesthesia/ventilator “pop-off” events -- "There are no pop-offs with POPSafe™!"
The global Endotracheal Tube Market was valued at $2.1 Billion in 2022 and is projected to reach a value of $3.1 Billion by 2030 with a compound annual growth rate (CAGR) of 5.9%. The US endotracheal tube market size is estimated to reach a value of $1.08 Billion by 2030 (35% of the global market size).
POPSafe™ Endotracheal Tube Sets are single-use prescription devices. The POPSafe™ Endotracheal Tube Sets will cost the same as current products and will be priced within the Medicare reimbursement price range for current standard of care endotracheal tube devices.
We have developed initial POPSafe™ prototypes and conducted successful initial simulated clinical use testing. POPSafe™ is a next-generation endotracheal anti-disconnect device, which will be GMP manufactured, and FDA 510(k) cleared, similarly to currently available endotracheal tube devices.
Video
-
Awards
-
 2023 Medical Honorable Mention
2023 Medical Honorable Mention -
 2023 Top 100 Entries
2023 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Kay Fuller
- Type of entry:individual