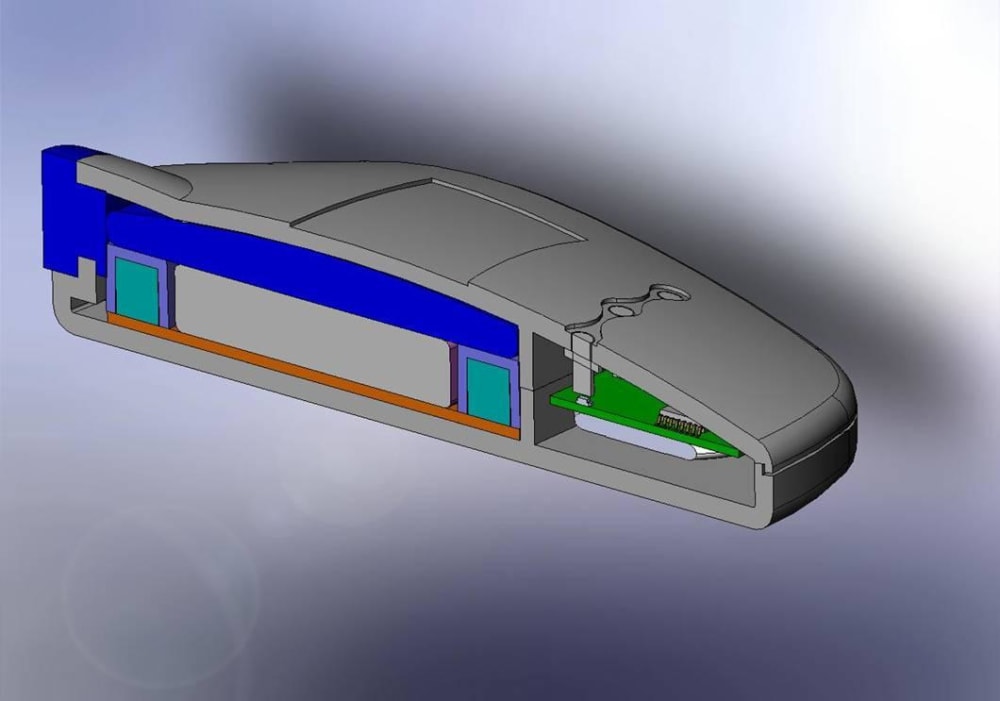
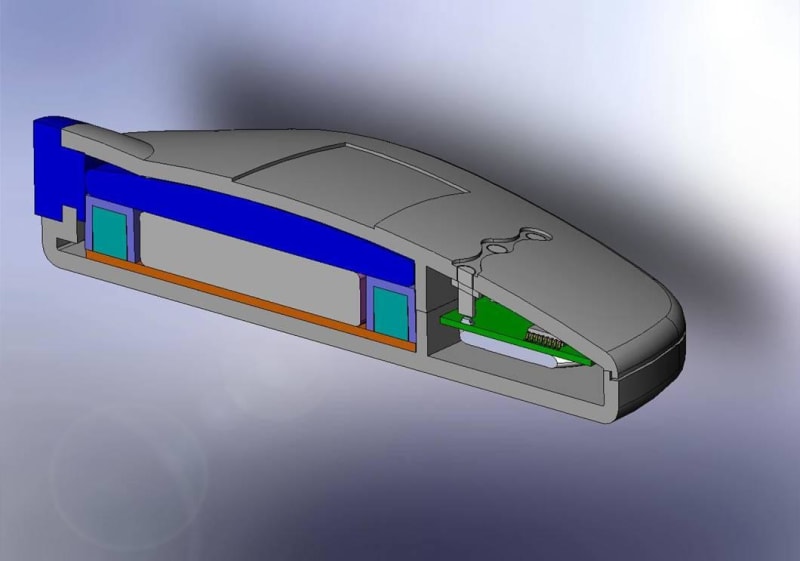
The Mini Infuser™ is a miniature, disposable, programmable drug delivery device designed to significantly lower the cost of patient care while improving a patients lifestyle with increased pharmacological safety, patient mobility and fewer needle sticks. Medipacs is currently developing the second generation ready for commercialization prototype shown in the images and will submit it to the FDA for approval once testing is completed.
Broad application of this technology will impact and lower the cost of healthcare not only for millions of infusion patients but also the industry providers. The projected market in the United States alone is greater than $3Billion. The estimated cost savings to US healthcare is over $1Billion annually once the product is implemented into just a third of the market. While the impact to poorer regions through out the world is immeasurable, as life improving drug therapies such as low cost continuous insulin delivery will be enabled and become available to patients within these regions for the first time.
The Medipacs Mini Infuser™ is a unique medical device that the electric motor and mechanical pump components have all been eliminated and replaced with a Smart Polymer Actuator that responds to changes in pH. The Actuator is controlled by a programmable electrical current that allows it to expand up to 500% of its original volume in order to accurately meter out a liquid medicine.
The first international patent has issued and there are 35 US and international patents pending that cover the device, materials, actuator, and component designs as well as use.
The Design and Engineering team had to overcome many obstacles of a never before developed technology in order to make new materials and methods of the following - epoxy polymer fabrication, actuator design and packaging, electrode materials, electrolyte chemistry, voltage and current control, circuitry and feed back loops, barrier materials, and mechanical engineering. All of this had to be cohesive and compatible for the device to be manufactured in a high speed wet processing line and once manufactured allow for the device to sit on a shelf for up to a year before patient use.
The team then had to reduce all of the components into the product package in order to create a commercial product. Over and above this the team has kept the cost of the device within the market specifications.
Like this entry?
-
About the Entrant
- Name:Mark Banister
- Type of entry:teamTeam members:Mark McWilliams, Mark Banister, Anthony Delizza, Mark Van Veen, Ray Clark, Gary Walters, Erich Coiner, Pat Murphy, Yordan Geronov.
- Hardware used for this entry:NI Lab View system with HP ComputersSoftware used for this entry:Solid Works
- Patent status:patented