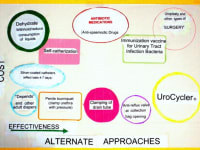
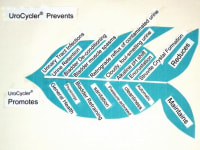
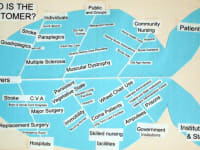
The UroCycler Automatic Bladder Management System serves indwelling catheterized patients as a magnetic prosthetic sphincter system which allows their bladder to function in a normal cyclic manner. This is important because, without the UroCycler, more than 99,000 American children and adults die each year from fatal catheter-associated urinary tract infections (CAUTIs) . The cost of this morbidity statistic to our USA healthcare system exceeds $1.9-Billion annually. Indwelling catheter users of all ages,race, gender, etc. are vulnerable to chronic urinary tract infections (UTIs), of which about 900,000 are reported in America each year. The medical bills for this problem exceed $10-Billion annually.
The key component, a unique, very low pressure-sensitive magnetic valve,is attached to the proximal end of the indwelling Foley catheter exiting the body. This device is precision made to critical tolerances, assembled in a clean room environment, and utilizes carefully- engineered inert ceramic permanent magnets to create a controlled attractive magnetic field strength to hold the valve closed initially. This stops the constant drip-drain traditional feature of the Foley-type catheter until a small pressure builds up within the filling bladder. When the urine pressure reaches a normal voiding value (about 20 cm height of a column of water), then the valve opens fully and the patient experiences a "normal" flow rate of urine exiting the body until the bladder is empty. In other words, this unique valve opens at a low, safe, appropriate pressure and remains open until the bladder is drained into a modified urine collection bag and there is no measurable fluid pressure remaining. This combination of performance features classifies the system as "hydrodynamically balanced" as well as serving as a positive unidirectional anti-reflux valve.
Clinical test and studies conducted in hospitals have proven that is operates, automatically, at little or no risk to the wearer, requiring only an occasional collection bag change for typically thirty days use, allowing users to sleep through the night and attend social events with dignity and an enhanced quality of life. In addition, users have experienced little or need to take expensive antibiotics, anti-spasmodics, pain killers, and other drugs which have undesirable side effects.
By restoring normal filling and flushing, the frequency and severity of CAUTIs have been observed to be PREVENTED and REDUCED by an amazing 90.9%
Patients also avoid dangerous catheter tip encrustation, unpleasant odors when urine turns from acid to alkaline, and buildup of sediment in the bladder (especially with MS patients).
It is important to note that this system has been accepted and used in 72 countries and has enjoyed approval for use in over 6,000 hospitals all over the fifty USA states.
Product quality audits prove compliance to achieve the CE Mark, the Theraputic Goods Administration, approval and seven types of US FDA Certifications. Three trademarks, seven US patents, and many foreign patents help to protect its proprietary design and performance features. This life-saving system has been awarded honors by the R & D 100 Competition, the Medical Excellence Gold Award, and the Invent America Competition.
-
Awards
-
 2014 Medical Honorable Mention
2014 Medical Honorable Mention -
 2014 Top 100 Entries
2014 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Dr. David Flinchbaugh
- Type of entry:individual
- Patent status:patented