We present a device that can provide safe, low cost cooling and heating without electricity, which can preserve food and medicine in remote environments, potentially saving the lives of up to 1.5 million children who die each year of vaccine-preventable diseases. It could also provide backpackers electricity-free heating.
The device is essentially a heat pump, transitioning from ambient temperature to warm or cold in a matter of minutes. It can keep vaccines cold by absorbing heat from an insulated container during its cooling phase, and releasing that heat outside during its heating phase.
How it Works
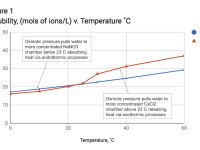
The device operates using two chambers separated by a semipermeable membrane, containing exothermic Calcium Chloride (CaCl2) on one side, and endothermic Sodium Nitrate (NaNO3) on the other. CaCl2 and NaNO3 were specifically chosen for several reasons: their saturated solutions have similar concentrations, their concentrations are temperature dependant, and the temperature/concentration lines cross in such a way that significant heating and cooling can be triggered simply by applying small temperature changes (Figure 1). Raising or lowering the temperature above/below 23°C makes one salt more soluble than the other, pulling water to it and causing significant heating or cooling (+/- 30˚C) in just a few minutes. Importantly, the dissolution process is reversible, so if a salt releases heat when it dissolves, then it absorbs the same amount when it precipitates, meaning both salts contribute to both heating and cooling.
Operation
To use the device for cooling (Figure 2), place it into a cool environment, slightly below 23˚C (e.g. bucket of river water or already chilled cooler). At this temperature, NaNO3 is more soluble than CaCl2 so the water will migrate to the NaNO3 chamber, beginning the cooling cycle. It can then be placed into an insulated box to protect food or medicine. It will absorb heat from the box. When the device is removed from the box, it warms to the outside ambient temperature. Above 23˚C the water migrates to the CaCl2 chamber, and the device becomes very hot, releasing heat absorbed from within the box. It is then allowed to cool to the ambient temperature, and is now ready to be used again.
For providing warmth in a cold environment, this process is reversed (Figure 3) and can be triggered with a small amount of heat (e.g. by holding close to your body or laying it in the sun).
Manufacturability
This device uses commonly available materials. Our prototype consists of a flexible, waterproof plastic bag, an inner bag made of a semipermeable membrane, CaCl2, NaNO3 and water. The NaNO3 is placed inside the semipermeable membrane bag which is sealed and placed inside the waterproof plastic bag. CaCl2 and water are added to the waterproof bag and then the waterproof bag is sealed.
The only expensive or difficult to manufacture component is the semipermeable membrane, which needs to be durable enough to survive repeated cycles. Similar membranes are commercially available in products retailing for around $50.
We've filed a Provisional Patent on this technology.
Like this entry?
-
About the Entrant
- Name:Mark Daly
- Type of entry:teamTeam members:Mark Daly
Nathan Daly
Connor Daly
Jesica Pedroza - Software used for this entry:Excel, Google Docs, Google Sheets
- Patent status:pending