Glaucoma is the second leading cause of blindness, affecting 80 million patients globally including 3 million patients in the USA. Elevated intraocular pressure (IOP) is an important risk factor in the pathogenesis and progression of glaucoma. It is caused by gradual blockage in the eye’s drainage channels that drain the aqueous humor (AH) from the eye’s anterior chamber (AC) resulting in pressure increase in the AC, optic nerve damage and blindness. Currently, a glaucoma drainage device (GDD) is implanted after medical treatments fail to control IOP by providing a conduit to drain AH from the AC to the sub-conjunctival/sub-Tenon space. Unfortunately, current GDDs have major drawbacks.
- Large sizes require lengthy and extensive surgical dissection.
- Large volume of AH is released abruptly causing serious vision threatening complications.
- Prolonged inflammation and fibrosis, lead to 50% premature device failure over 5 years.
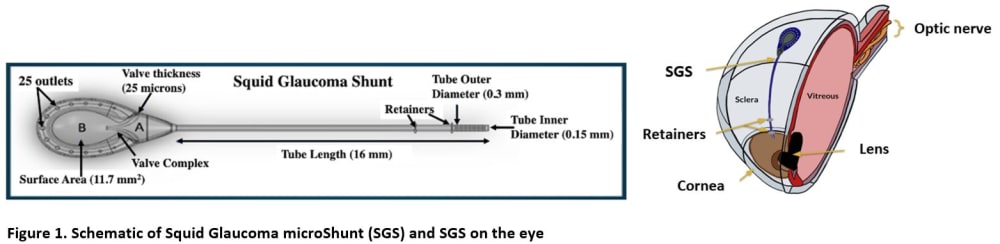
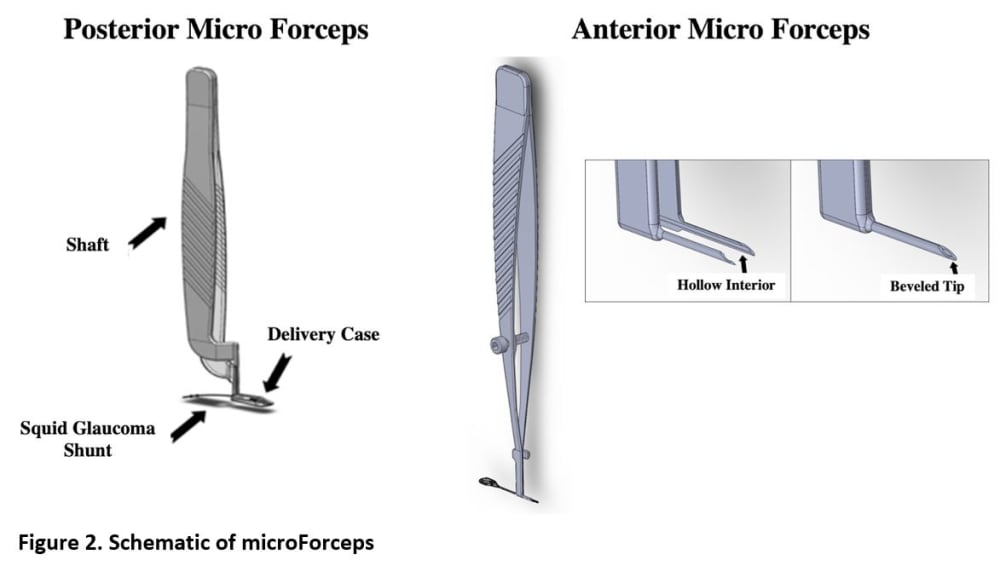
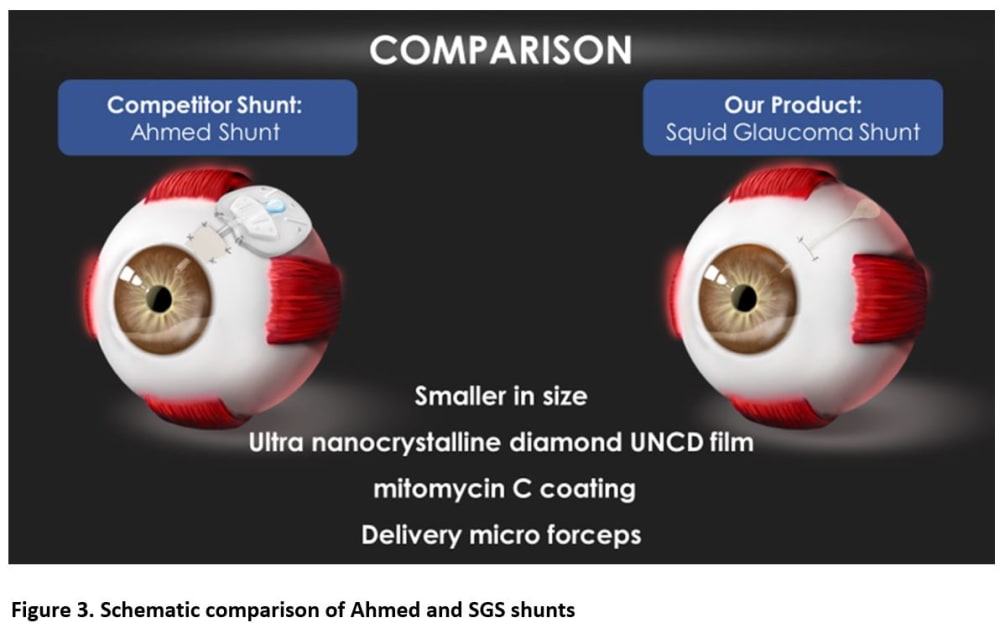
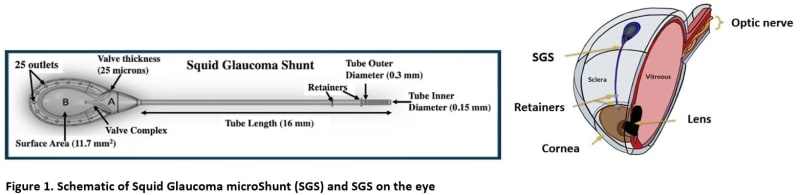
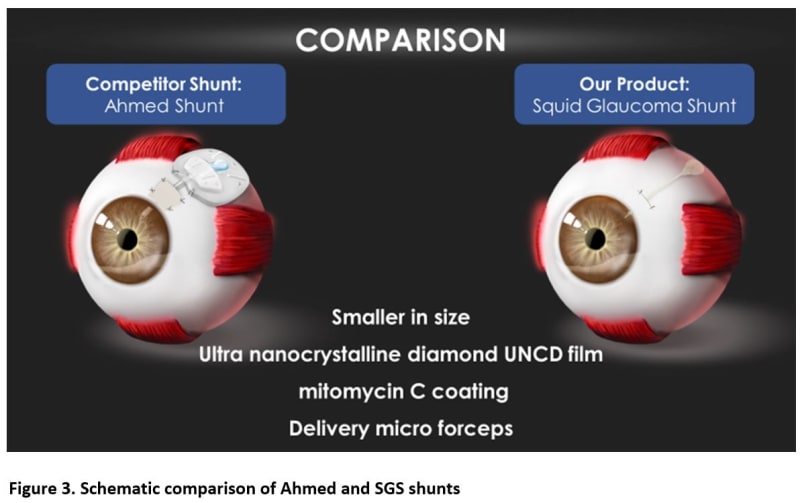
By comparison, the proposed Squid Glaucoma microShunt (SGS) will be 90% smaller and will provide a slow and controlled release of AH through a narrow tube and then further slowed by a channel placed between the chambers (Figure 1). Thus, the SGS would require minimal surgical dissection and the AH will be released in a slow sprinkle-like fashion, minimizing inflammation, fibrosis, and complications. Two built-in retainers would provide stability, eliminating the need to suture the device to the sclera. Current GDDs are made of materials that provoke inflammation and fibrosis often leading to intense encapsulation and elevated IOP. The SGS device will be coated with a patented low-cost ultrananocrystalline diamond (UNCD) film which exhibits super-hydrophobicity and superb biocompatibility. Finally, a pair of patented micro forceps (figure 2) would facilitate quick and easy insertion of the SGS promoting a 50% reduction in surgery time enabling surgeons to perform faster and more efficient surgeries (weblink 1).
In the USA alone, direct costs related to glaucoma reached $ 5.8 billion annually according to a 2013 report. The global glaucoma surgery market was valued at $818 million in 2020, projected to reach $6.5 billion by 2030 with a compound annual growth rate of 24.6%. At present, due to the complexity of the procedures, complications, and invasiveness of the bulky GDDs, only 40% of glaucoma patients undergo surgical interventions. The less invasive SGS implant would make early surgical intervention an option to wider sections of the glaucoma population with presumably better outcomes, thus preventing blindness.
Preliminary in silico fluidic simulation has showed that the SGS has the potential to significantly reduce IOP by 50% (weblink 1).
Additionally, the efficacy of the UNCD coating on reducing fibrosis has been tested and confirmed.
A SGS prototype will be manufactured in collaboration with world-renowned material scientists and engineers with extensive experience in micro-medical devices at the University of Texas at Dallas and at The University of Notre Dame. Two fabrication processes, hybrid low-cost 3D conformal printing and photolithography will enable the SGS to be cost-effectively produced.
Video
-
Awards
-
 2022 Medical Category Winner
2022 Medical Category Winner -
 2022 Top 100 Entries
2022 Top 100 Entries -
 2022 Top 10 Most Popular
2022 Top 10 Most Popular
Like this entry?
-
About the Entrant
- Name:Karanjit Kooner
- Type of entry:teamTeam members:Karanjit S Kooner MD PhD
Orlando Auciello PhD
Walter Voit PhD
Yanliang Zhang PhD - Software used for this entry:ANSYS
- Patent status:pending