OBI-USA/OBI-México developed a NEW TRANSFORMATIONAL commercial metallic dental implant coated with a unique best biocompatible (because made of Carbon atoms-element of life in human DNA, cells, molecules) / body fluids corrosion resistant / LOW-COST production Ultrananocrystalline Diamond (UNCD) coating. OBI-USA/OBI-México are the only companies worldwide developing UNCD-coated medical implants, with seventeen strong patents on the UNCD coating technology.
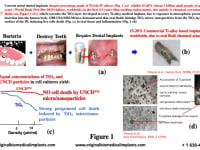
Current metal dental implants (Fig. 1(a)) (mainly made of Ti-6Al-4V alloys) exhibit 15 % (0.5 billion adult people 15-64 years old / World Bank Web Site-2019) failure, worldwide, in the first 4-5 years (needing replacement), due mainly to chemical corrosion by oral fluids (Figure 1(b)), which corrodes the TiO2 layer developed in every Ti-alloy medical implant, due to atmospheric exposure, before implantation. OBI-USA/OBI-México demonstrated that oral fluids dislodge TiO2 micro/nanoparticles from the TiO2 layer on the DI’s surface, inducing live cells death (see Fig. 1 (c)) in oral tissue and inflammation (Fig. 1(d))
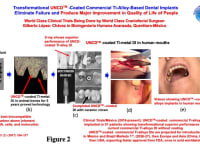
Commercial Metal (Ti-alloy) dental implants (DI) coated with best biocompatible/oral fluids corrosion resistant/low-cost UNCD coating eliminate implant failure by chemical attack from oral fluids, as in current commercial metal implants. UNCDTM-coated DIs (Fig. 2 (a)), developed by OBI-USA/OBI-Mexico, have been tested in animals for five years (Fig. 2 (b)), showing no chemical attack by oral fluids, exceptional osteointegration in bone, and no deleterious biological effects, as for commercial Ti-alloys (see Auciello et al, J. Biomed. Mater. Res.-B: Applied Materials, 008 (2016)). Clinical trials, implanting UNCDTM-coated commercial Ti-alloys DIs, in 51 male and female patients since 2018 (see Figs. 2 (c), 2 (d) and 2 (e)), in México, are showing transformational superior performance, compared to current commercial Ti-alloy DIs. The projection is inserting UNCD-coated DIs in the Mexican market in 2022, then Latin America, Europe, Asia, by 2023-2024, and finally the USA, to get faster FDA approval. The focus on UNCD-coated dental implants (DI) as the first medical product is because we have proven we can coat about 300 DIs (7-8 mm long x 2 mm diameter) in the industrial microwave plasma chemical vapor deposition (MPCVD) system currently operating in OBI-México (Fig. 3), in a single simultaneous coating process to enable low fabrication cost (projected ≤ U$S 3 per DI).
The DIs’ main world’s market addressed includes the USA (U$S 3 billion), Latin America (U$S 0.5 billion), Europe (U$S 3 billion), China (U$S 0.5 billion), and India (U$S 2 billion), Asian Pacific (U$S 1 billion)
Other UNCD-coated medical implants under development include the following: 1) UNCD-coated stents, with the coating eliminating thrombus formation due to super-hydrophobic surface, inhibiting blood cell adhesion as it happens in current metal-based stents; 2) UNCD-coated artificial hips and knees, with the coating eliminating current failure of metal implants, via body fluids’ chemical attack, and providing superior resistance to mechanical wear, due to UNCD coating exhibiting the smallest friction coefficient (~ 0.02) of any materials used today in artificial prostheses, and with practically no mechanical wear, since UNCD coatings exhibit all mechanical properties of natural diamond, the strongest material known on Earth.
Like this entry?
-
About the Entrant
- Name:Orlando Auciello
- Type of entry:teamTeam members:
- Gilberto Lopez-Chavez
- Daniel Olmedo
- Debora Tasat
- Orlando Auciello
- Patent status:pending