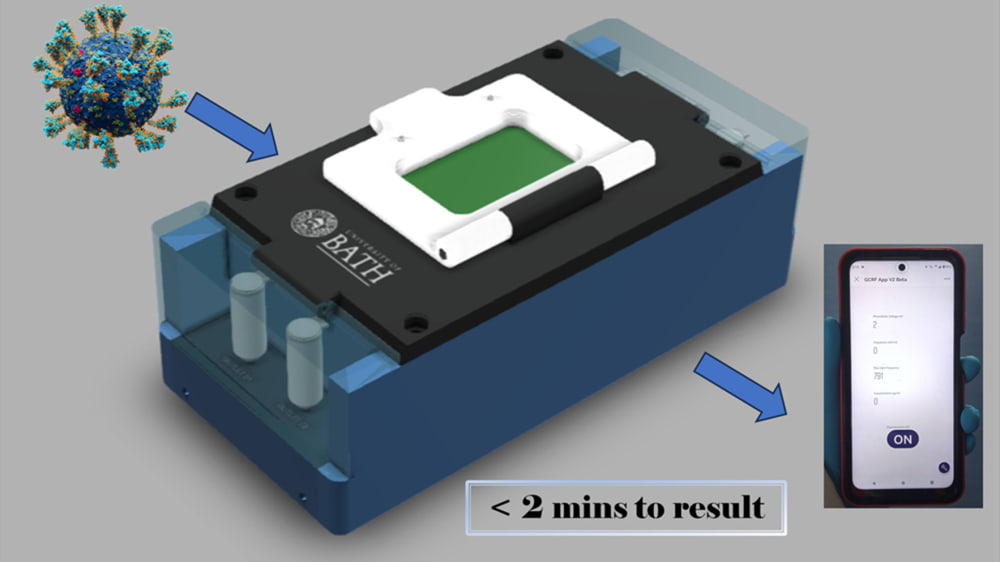
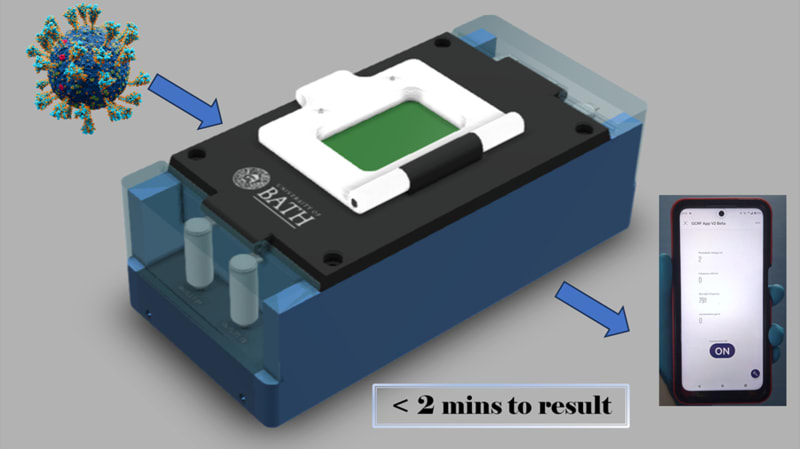
LoCKAmp is the first commercially manufactured, miniaturised, printed circuit board (PCB)-based device for loop-mediated isothermal amplification (LAMP) genetic detection of pathogens. The need for more accurate monitoring of the spread of infectious diseases in the community was emphasized by the recent COVID-19 pandemic. The lack of diagnostic tests suitable for point-of-care (PoC) applications during the initial pandemic stage, along with the sensitivity limitations of the only test which has been used outside laboratory settings (i.e. the lateral-flow tests), led to ineffective monitoring of the virus transmission. The gap between the rapid but low-sensitivity lateral-flow tests and the sensitive but expensive and time-consuming nucleic acid amplification tests (NAAT) has yet to be addressed. Lab-based NAATs are predominantly polymerase chain reaction (PCR)-based assays, the gold standard, but requires complex and time-consuming sample processing. Access to handheld and affordable NAAT would render the healthcare response prompt and precise in both developed and, crucially, low-resource settings with limitations in centralized analytical capacity.
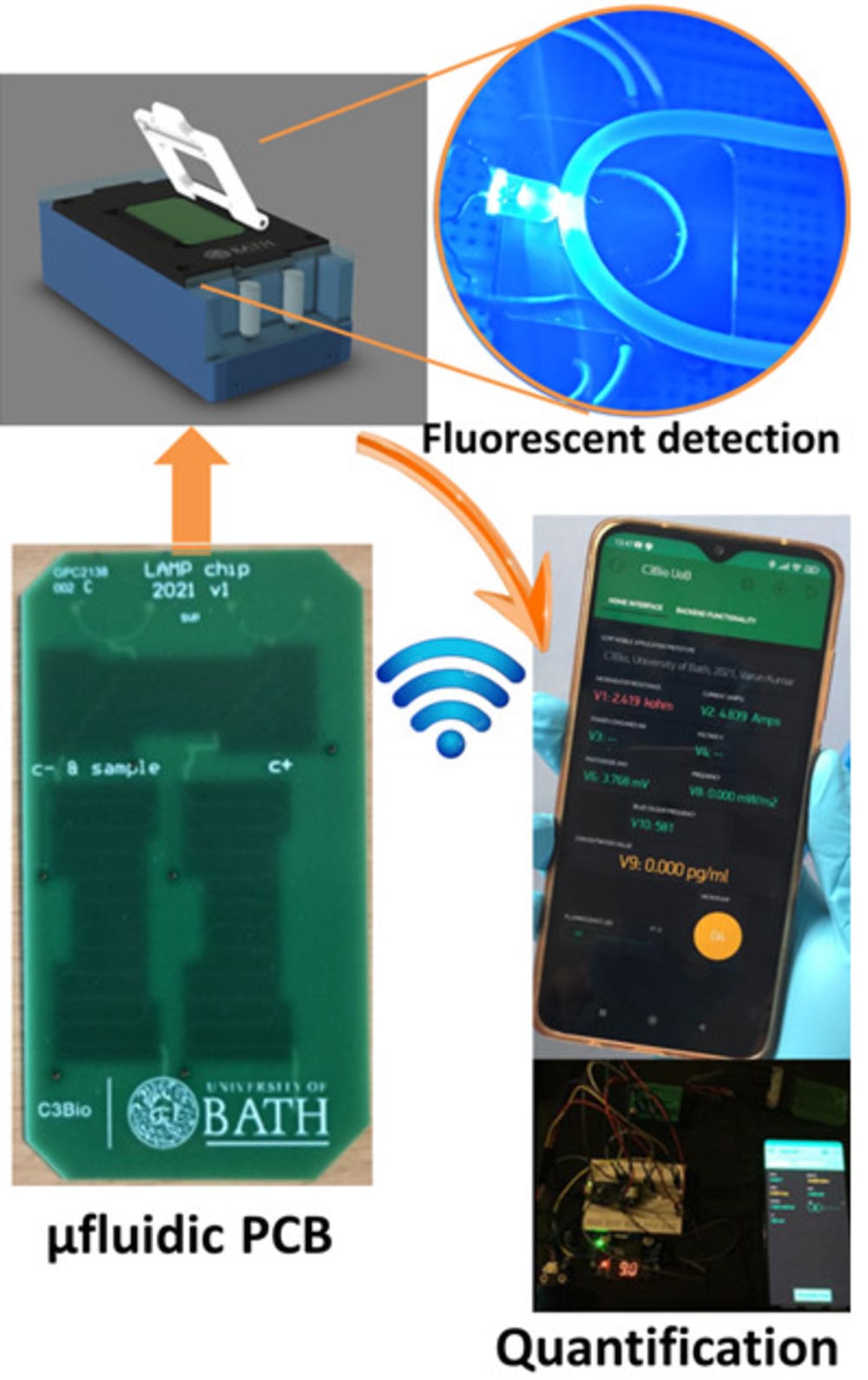
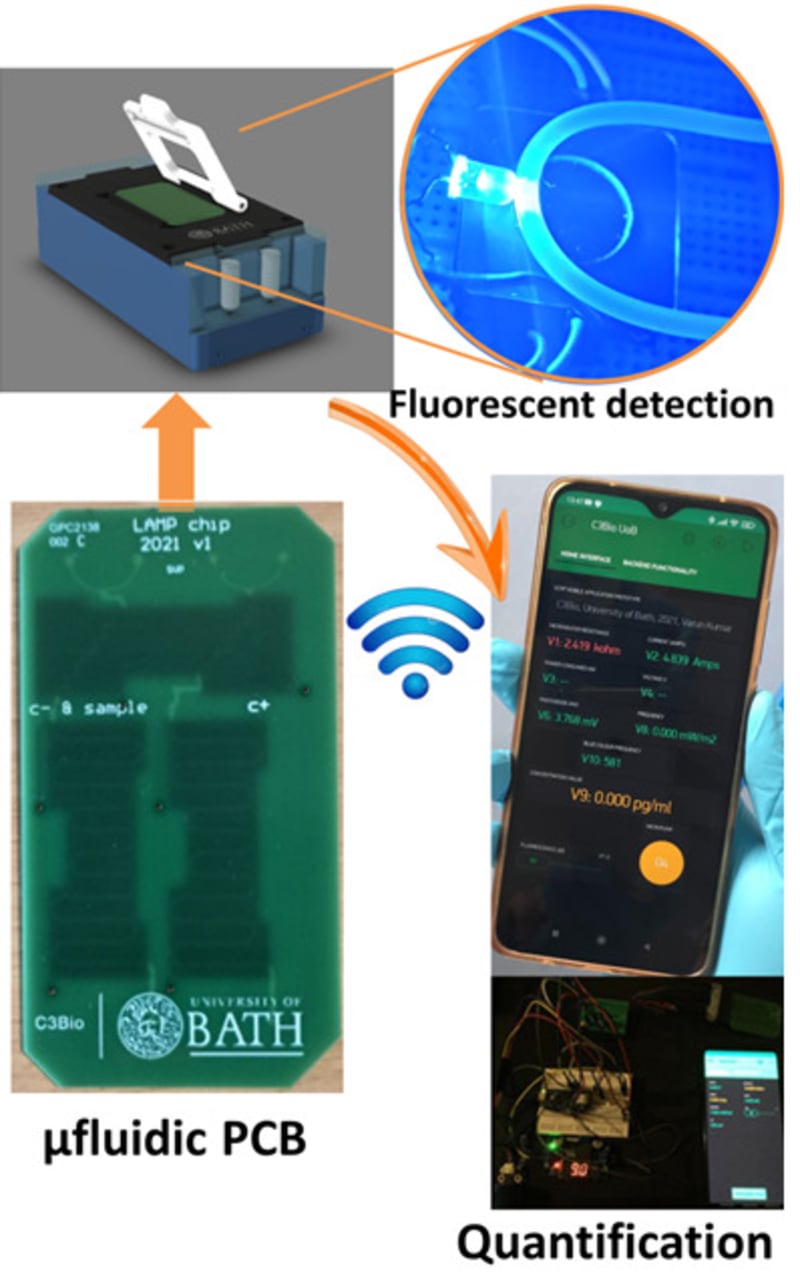
The true innovation of LoCKAmp lies in its unique electronics integration with microfluidic channels which enables straight-forward up-scalability. LoCKAmp leverages PCB technology and manufacturing to create a low-cost, continuous-flow μLAMP platform with off-the-shelf optical detection components. It uses a mass-manufactured PCB chip with fluorescent detection circuitry for virus detection, providing care providers and public health bodies with a new diagnostic tool. The device amplifies genetic material from a nasal swab via a chemical reaction, with results viewable on a smartphone app. Liquid is pumped through tiny, transparent, ‘microfluidic’ channels embedded in the PCB, above copper heaters just 0.017- mm thick, heating the sample to release RNA, which RT-LAMP chemicals then multiply. The team says LAMP detection is preferable to PCR as it is more sensitive, faster and more specific. A further benefit of the design is that no pre-processing of the nasal swab samples is required. If the specific virus RNA is present in the amplified sample, it fluoresces under light — this signal is then used to denote a positive test. The device was tested with COVID-19 patient swabs collected by Bath’s Royal United Hospital Trusts during the third wave of the pandemic.
Ultrafast, SARS-CoV-2 RNA amplification was demonstrated within 2 min analysis, at concentrations as low as 17 gene-copies μL−1 in wastewater samples. This renders the presented miniaturised NAAT diagnostic test the fastest reported SARS-CoV-2 genetic detection platform, in a practical implementation suitable for deployment in the field. The research team said the technology could easily be adapted to detect other pathogens such as bacteria — or even conditions like cancer.
LoCKAmp chips are currently mass-manufactured in a commercial, ISO-compliant PCB factory, currently made for £2.50 ($3.10) and could cost less than 50 pence ($0.62). The testing unit is projected to cost as little as £50 (about $62) when it reaches mass production. The research team said a commercial partner with the relevant design and manufacturing expertise could quickly redesign the LoCKAmp into a small, portable device — with great potential for use in remote healthcare settings.
-
Awards
-
 2024 Top 100 Entries
2024 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Sotirios Papamatthaiou
- Type of entry:teamTeam members:
- Sotirios Papamatthaiou
- Despina Moschou
- Patent status:none