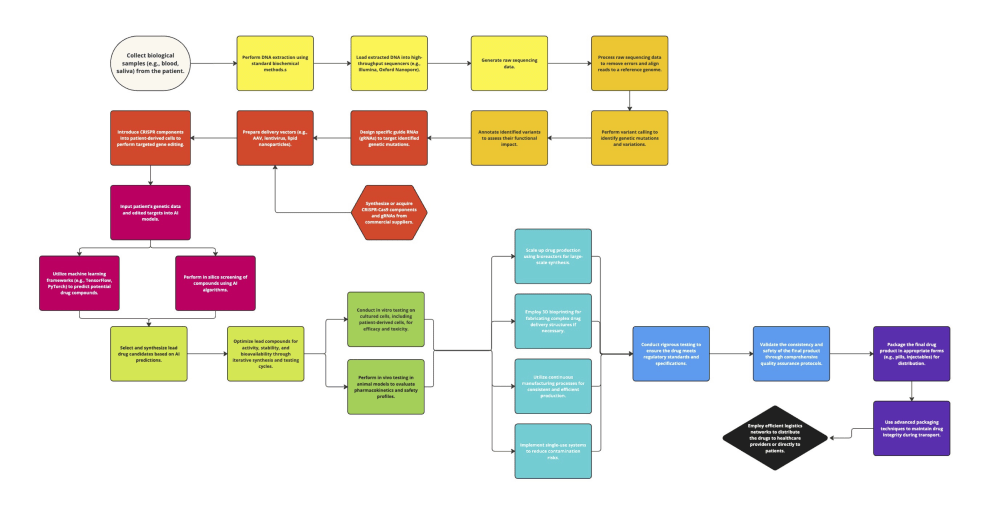
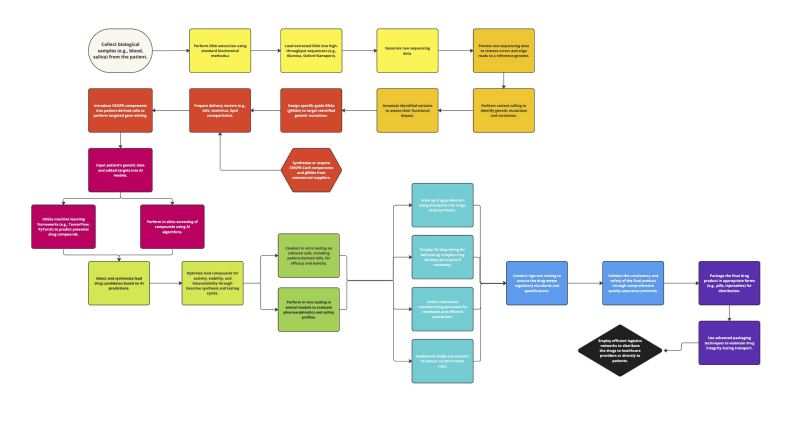
Tailored medical treatments represent the advent of individually-tested therapeutic interventions, providing optimal efficiency with minimal side effects. A new technique combines CRISPR gene-editing technology and artificial intelligence-guided drug design to create drugs specifically designed for the genetic makeups of individuals. CRISPR-Cas9 is a targeted genome editing technology that can be used to fix mutations: the incorporation of artificial intelligence (AI) provides enormous computing power and the capability to detect patterns in drug design. Together, they offer a potent framework for creating highly personalized and effective drugs designed to match the genetic blueprints of each unique individual.
The researchers developed a three-staged solution: deep genetic sequencing, CRISPR gene editing, and drug design execution using AI. Building a system to design personalized drugs with CRISPR and AI starts with biological samples from patients being sequenced through genetic sequencing, which reads the patient's genome. Sequencing data is then analyzed to find genetic variants using bioinformatics tools. Sequence-specific guide RNAs (gRNAs) are used to target mutations; they then move the CRISPR-Cas9 complex along for targeted gene modification. These can then be used for in silico modeling to predict different drug compounds, whose biological activity is confirmed through both in vitro and early animal model testing; since it leverages pre-existing genetic sequencing and CRISPR infrastructure rather than requiring extensive new equipment; this makes for a much more efficient production method.
This cost-effective, functionality-based hardware production relies on biocompatible materials, such as polycarbonate (PC), for interfacing with biological samples to build the personalized drug design system using CRISPR and AI; it implements robust data management, using programming languages - like Python - and frameworks - such as TensorFlow or PyTorch for advanced data processing for AI. It consists of large-scale bioreactors for high-throughput volume production, 3D-bioprinting to rapidly develop complex drug-delivery systems, and continuous manufacturing operations designed to increase overall efficiency and consistency: single-use systems will control contamination risks. To ensure high-quality standards, the final product is packaged and delivered through a resilient logistics network for safe delivery directly to patients.
Personalization of treatment for genetic disorders such as cystic fibrosis, sickle cell anemia, and certain cancers leads to higher efficacy with lower side effects, improving public health. It is fine-tuned for the care of chronic diseases—each year, 5.9 million people die from conditions that could be helped with pharmaceuticals (World Health Organization). It functions optimally in countries with scarce access to accurate therapies, mostly in tropical locations such as Sub-Saharan Africa as well as some regions of Southeast Asia, and Latin America. Yet these regions tend to rely on broad-spectrum drugs that can lead to drug resistance and have serious side effects: this already provides a huge market, with the pharmaceutical segment expected to reach almost 400 billion in global sales by 2024. Here, personalized drug design may significantly improve treatment outcomes and reduce inequality among these marginal zones of therapeutic interventions.
-
Awards
-
 2024 Top 100 Entries
2024 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Sepehr Khavari
- Type of entry:individual
- Patent status:none