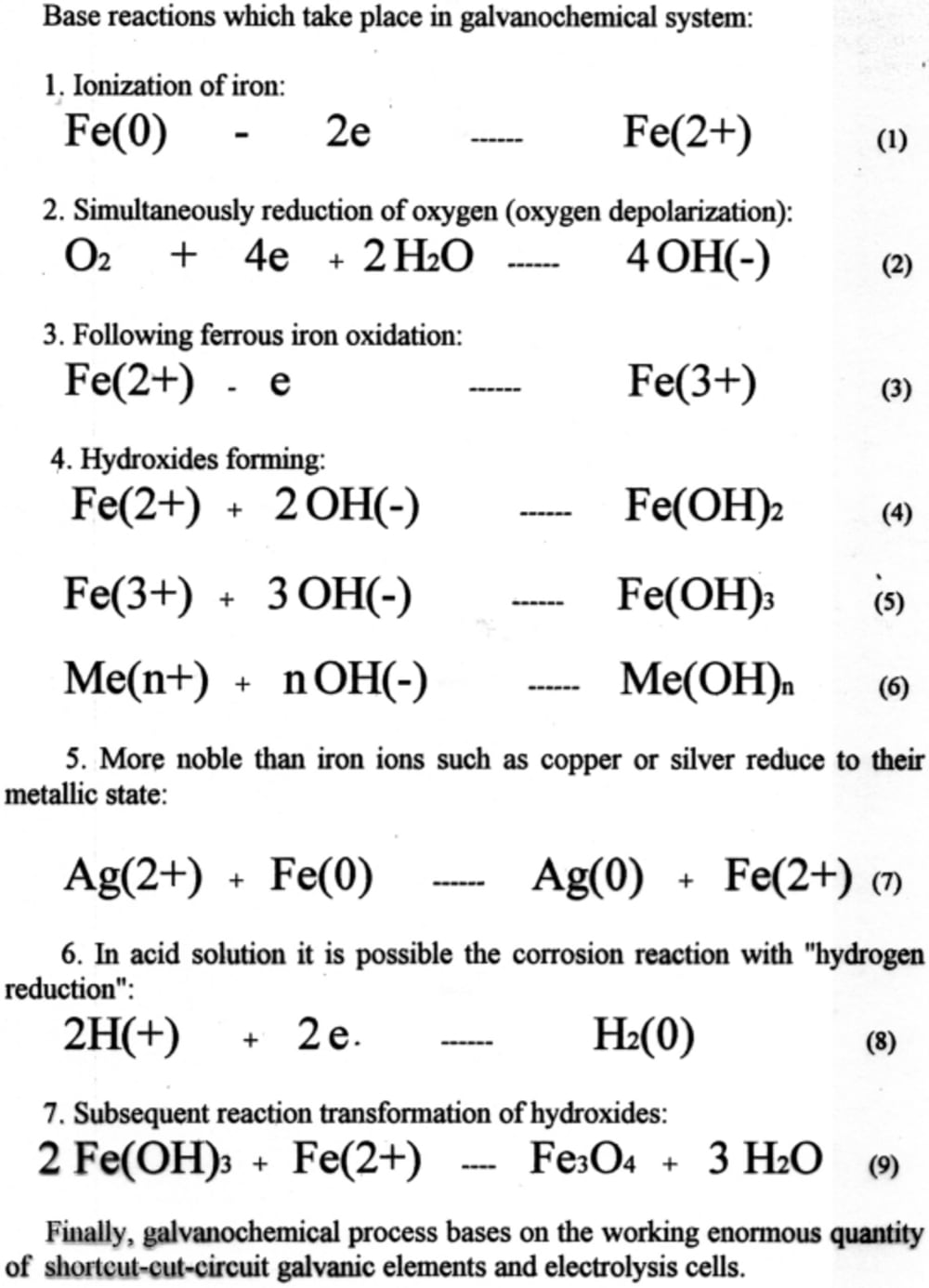
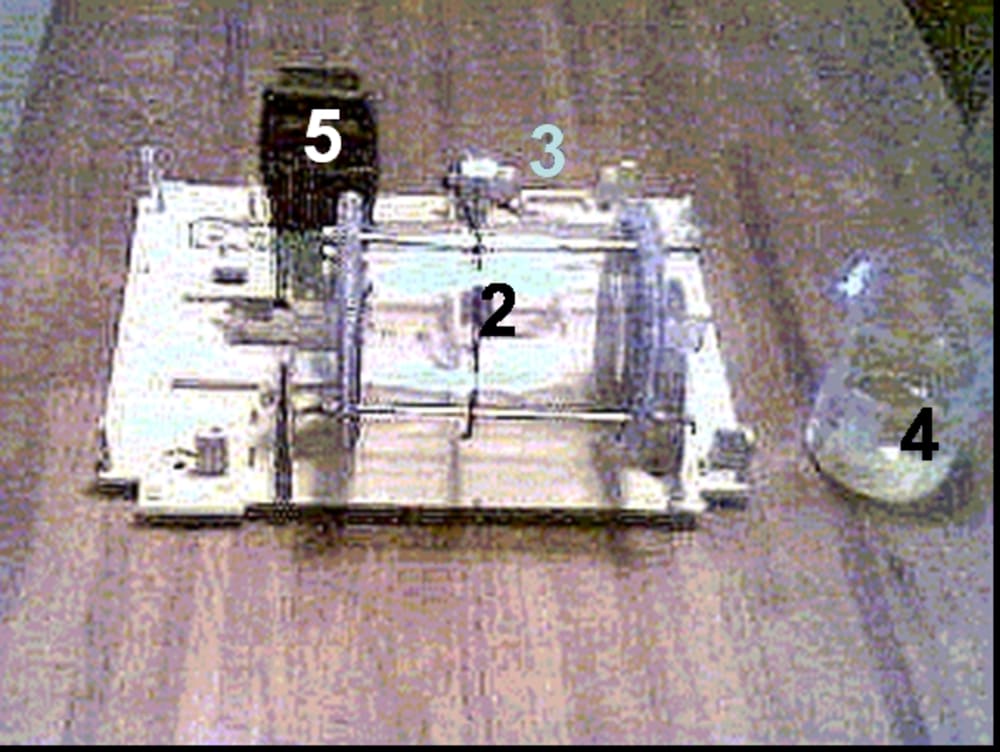
Galvanochemical process can be used for water treatment and decontamination, as well as for production of a vast rage of chemicals and compounds, such as oxides, hydroxides, salts, etc.
One of the driving forces in the galvanochemical process are ferrous and ferric ions, because they are responsible for transforming chromium ions (6+) to (3+), and precipitating all admixtures from the solution.
Galvanochemical (galvanocoagulation) process uses carbon steel’s oxygen corrosion for producing ferrous ions. The spontaneous corrosion process involves iron and oxygen from air in basic, neutral and weak acid solutions. As results, in case of acid solution its pH after treatment increased, and in case of bases solutions its pH decreased. Beside this, byproducts of oxygen reduction and iron oxidation such as hydrogen peroxide, hydroxyl radical and hydroperoxyl radical can degrade and volatilize organics via oxidizing or hydroxylation. Noble organic compounds, which non react with corrosion byproducts, especially surfactants, adsorb on hydroxides and removed from the solution.
Finally, galvanochemical process has in it basis simultaneous working enormous quantity of shortcut-circuit galvanic elements and electrolytic cells. Contaminant removal takes place by the combined action of homogeneous degradation of organics with oxidizing radicals and their coagulation with hydroxides. Other significant advantage of this process in comparison with the reagent method is that treatment doesn’t increases amount of salts in treated water. Kinetics of the steel corrosion, along with its sequence of the hydroxides forming, determines the process of wastewater decontamination in its base.
Forming during galvanochemical process products and compounds have unique properties such as particles size, structure, purity, and can be used in many branches of industry.
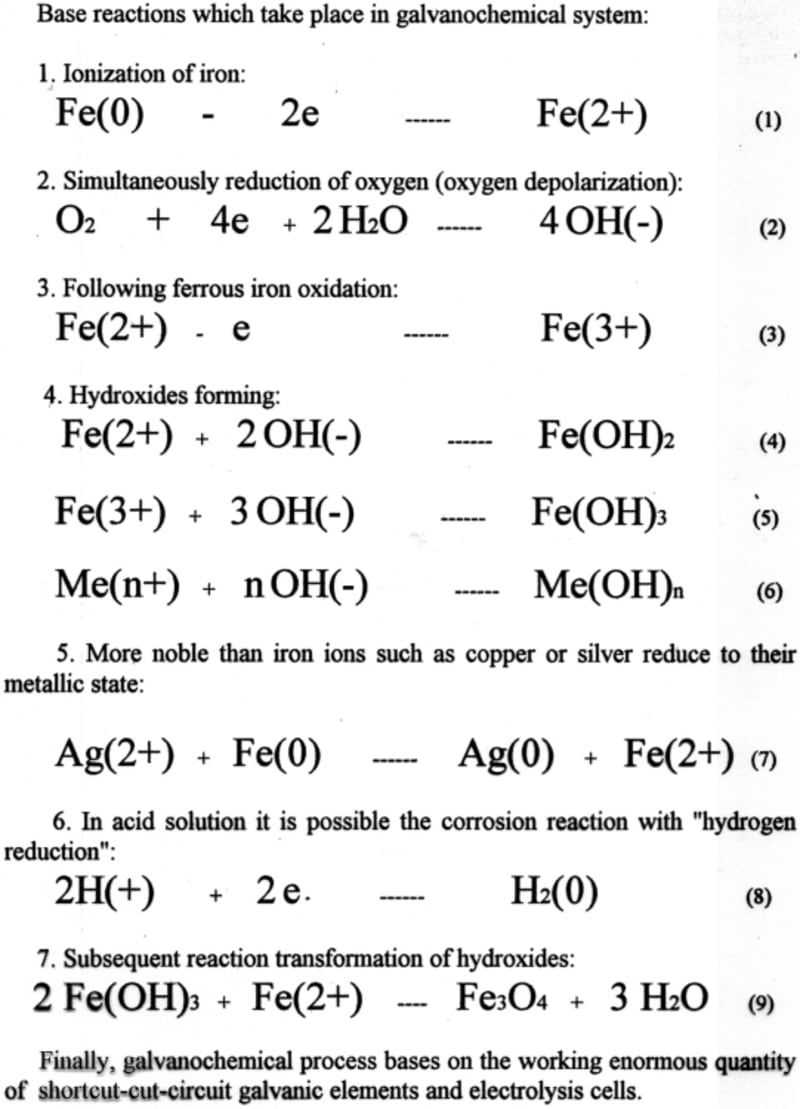
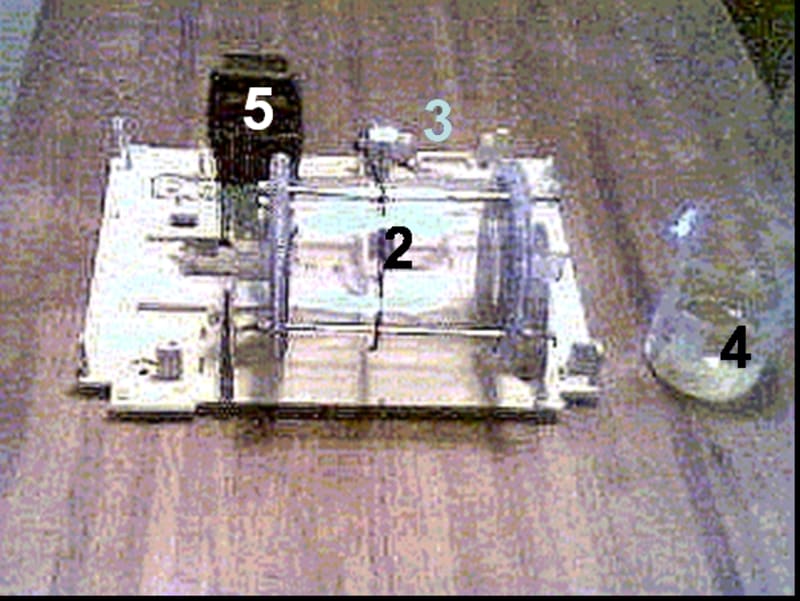
Equipment for this process contains a horizontal revolving cylinder with corroding material. That cylinder has inside mixing partitions (tumbling ribs), which are arranged in a so-called "waterfall" mixing pattern. For acceleration of oxygen reduction, which is linked to iron ionization, some neutral materials such as coke, titanium or stainless steel may be added to the galvanochemical system.
We tested laboratory set-up equipment for this process, and investigated some technological parameters of galvanochemical process on kinetics of carbon steel corrosion and hydroxides formation rates.
Experiments for removing neutral organic surfactants from modeling wastewater show efficiency of this process.
Like this entry?
-
About the Entrant
- Name:Aleksandr Pikelny
- Type of entry:individual
- Hardware used for this entry:Electrochemical and basic chemical equipmentSoftware used for this entry:MS Office
- Patent status:none