NASA’s Jet Propulsion Laboratory has developed a next generation, direct-feed fuel cell that is cleaner, cheaper, and more efficient than existing fuel cells. Methanol fuel cells use an oxidation/reduction reaction to generate electrical power without polluting the air or burning non-renewable fossil fuels. This reaction requires equal amounts of methanol and water, which makes conventional fuel cell vehicles impractically heavy. JPL’s methanol fuel cell is able to recycle the water that is used during its reaction, so a vehicle powered by JPL’s fuel cell would be lighter and more efficient. In addition, while state-of-the-art fuel cells use an expensive platinum catalyst to drive their chemical reactions, JPL’s fuel cell uses a more efficient catalyst that almost entirely eliminates the need for platinum. This fuel cell is ideal for vehicles and other portable applications because it permits the use of high-performance alternative fuels that can be stored and transported with ease.
THE TECHNOLOGY
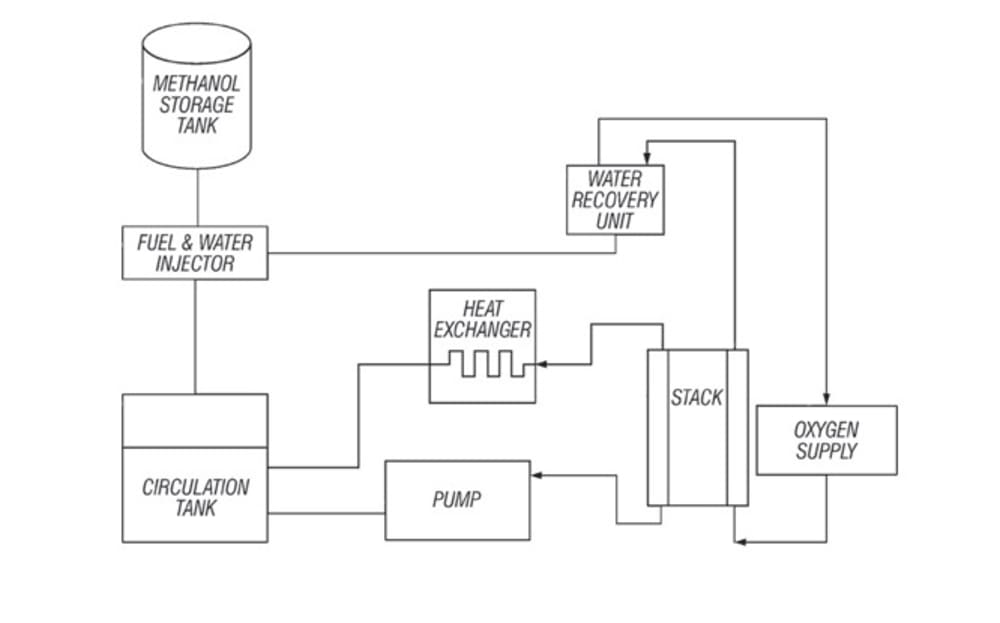
JPL’s direct-feed methanol fuel system comprises a membrane-electrode assembly, a
fuel storage tank, a water recovery unit, and an air supply unit. During operation, an
oxidation/reduction reaction occurs when the aqueous fuel solution is circulated past the anode while air is supplied at the cathode. A direct-feed design eliminates the need for a fuel vaporizer and its associated heat source, control system, and added mass. A solid polymer electrolyte membrane eliminates the need for a free soluble acid or base electrolyte and reduces fuel crossover, a parasitic reaction that lowers efficiency and generates heat in the fuel cell. A water recovery unit recycles the water used in the reaction and eliminates the need for a substantial water supply.
BENEFITS
* Direct-feed fuel cell eliminates the acid-induced corrosion that plagues
conventional fuel cells
* Improved catalyst eliminates the need for expensive platinum
* Water used in the oxidation/reduction reaction can be recycled from the cathode
* High-performance electrodes can be fabricated quickly and efficiently
PUBLICATIONS
U.S. Patent 7,488,548
-
Awards
-
 2015 Electronics Honorable Mention
2015 Electronics Honorable Mention -
 2015 Top 100 Entries
2015 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Subbarao Surampudi
- Type of entry:teamTeam members:Subbarao Surampudi, Harvey Frank, Sekharipuram Narayanan, William Chun, Barbara Jeffries Nakamura, Andrew Kindler, Gerald Halpert
- Patent status:patented