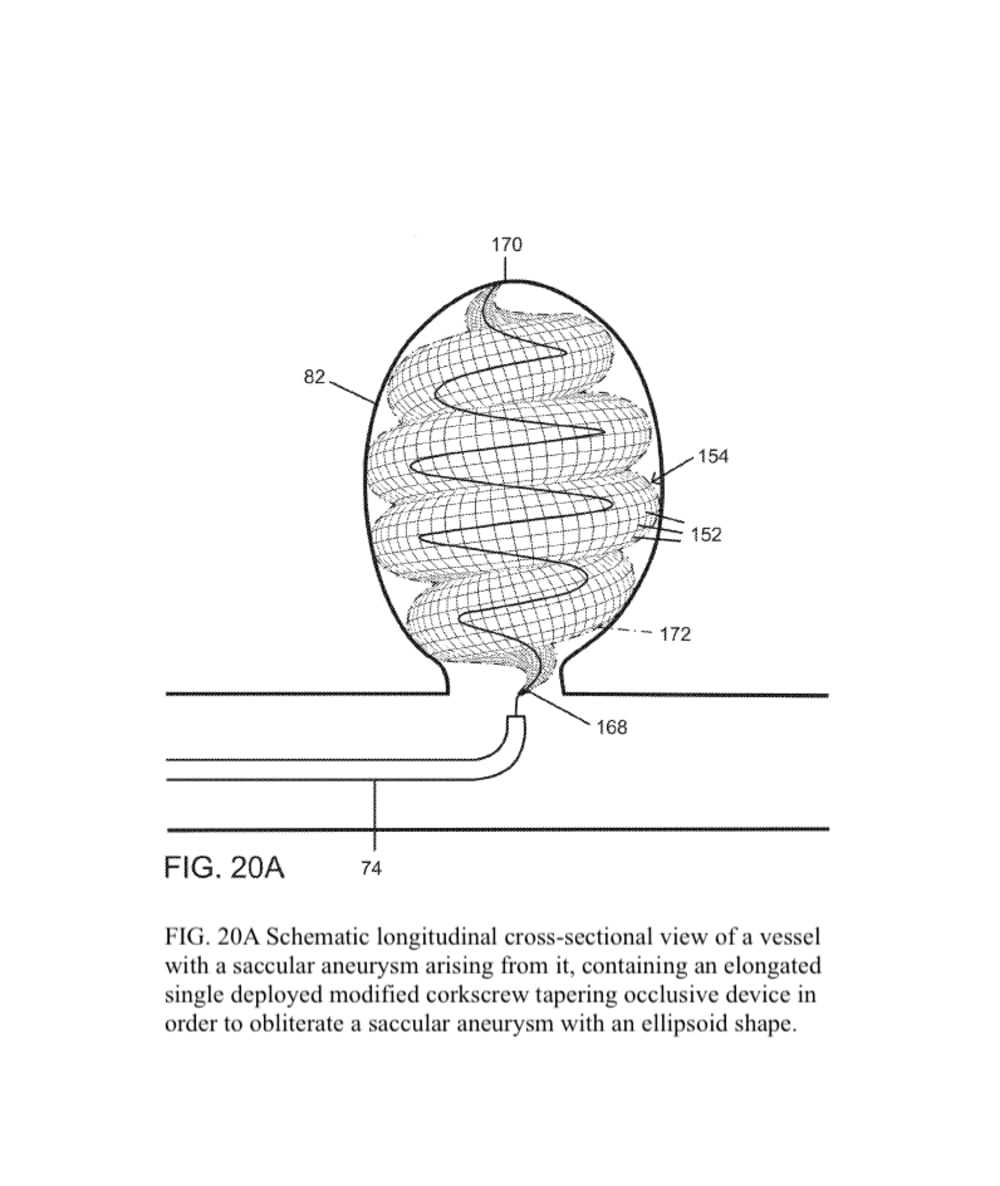
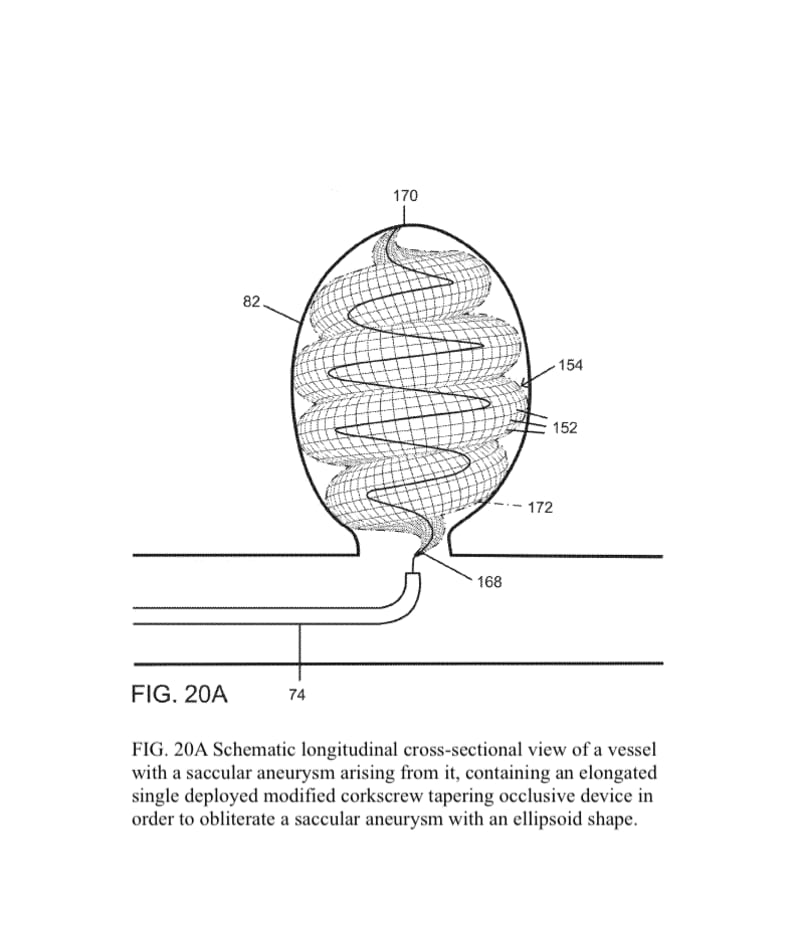
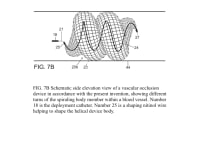
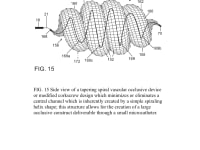
The present invention provides an improved vascular occlusion device and an associated surgical method which allows for primary treatment of saccular intracranial or peripheral aneurysms. Two covered or uncovered interlocking or intertwined helical devices may be assembled within the aneurysm sac to generate aneurysm occlusion and thereby sparing the parent vessel. Obliteration of the aneurysm sac may alternatively be accomplished with a single modified expanding occlusion device, which is tapered at both ends, in essence exhibiting a tapering tubular corkscrew design along both the leading and trailing ends of the occlusive device, thereby approximating a spheroid or ellipsoid shape typical of an aneurysm sac. The device outer wall for this purpose could be uncovered, covered with a microporous membrane, or covered with an impervious membrane.
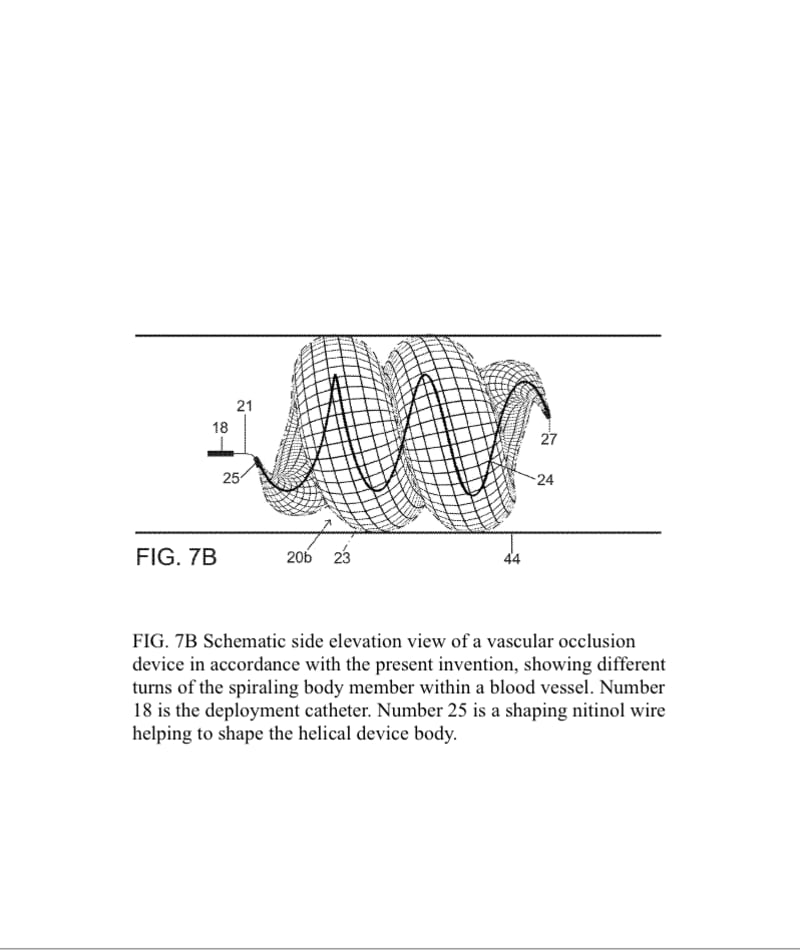
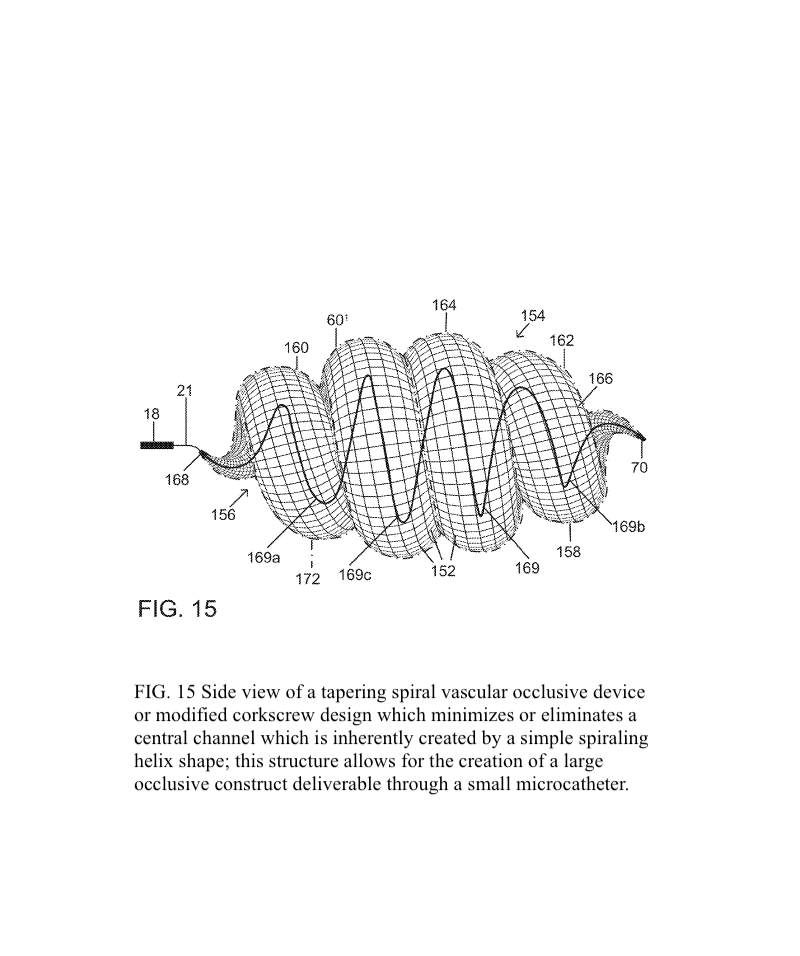
A vascular occlusive device comprises a superstructure expandable from a collapsed percutaneous insertion configuration to an expanded deployment or use configuration. The superstructure is comprised of both primary and secondary three-dimensional shapes allowing for the occlusion of a wide range of vessel sizes from small to large through a disproportionately small delivery catheter. The vascular occlusive device includes a shape memory element for the generation of radial force and the creation of the larger secondary three-dimensional twisting or helical superstructure as is needed for target vessel occlusion.
The advantage for treating ruptured cerebral aneurysms over current devices is related to its ability to get large relative to the small delivery catheter needed to traverse the brain arteries based on the principles of medical origami. The occlusive device, in essence, is assembled within the aneurysm sack, enabling it to assume a larger shape than current devices; this enables the treatment of larger than 10 mm aneurysms, the upper size limit for available intra-aneurysmal flow disrupters. Perhaps up to 20 percent of aneurysms are too large for current intra-aneurysmal flow disrupters.
In addition, the commonly used Woven EndoBridge (WEB) aneurysm embolization system has a high rate of cerebral aneurysm neck recurrences, up to 30 % with complete occlusion rates of 50-55 % and a cumulative re-treatment rate of 11 % at 3 years. The spiraling 3D tubular design would allow for a higher surface area or volume of intra-aneurysmal flow disruption relative to the sac-like WEB device with the potential for less compaction and a lower incidence of aneurysm neck recurrences.
The superstructure of the vascular occlusive device would be manufactured in a similar process to conventional vascular stents with shape memory wires such as nitinol. The production process begins with a nitinol wire being wound around a specific jig to create the device skeleton, resulting in a knitted structure. The braided design allows for flexibility and conformability. Radiopacity can be varied by increasing proportion of platinum-tungsten wires.
The device would be primarily marketed to vascular neurosurgeons for the treatment of ruptured cerebral aneurysms, and also to peripheral vascular surgeons for the treatment of abnormal blood vessels including aneurysms in the remainder of the body.
Like this entry?
-
About the Entrant
- Name:John Demeritt
- Type of entry:individual
- Patent status:patented