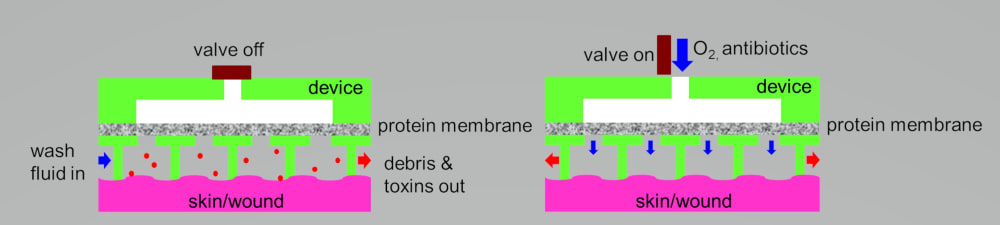
Current wound care and treatments require the removal of dressings, resulting in further disruptions to the surgical site or wound bed that can lead to severe discomfort, compromised healing and other complications. New objective approaches for monitoring and treating wounds are needed to improve surgical outcome and wound healing for both military personnel and civilians.
To address these needs, a transparent wound dressing is developed that provides unique real-time tissue oxygenation and other parameters across entire wound site for direct, continuous monitoring of tissue health throughout the healing process. A potential approach to eliminate the need for dressing removal during treatment is a therapeutic release system integrated into the bandage for interactive, spatio-specific delivery of drugs, growth factors, electrolytes and nutrients directly to vulnerable tissues. This artificial skin could be utilized to quantitatively measure and monitor fluid losses, and other modalities needed. In addition, these barriers /bandage system could be used for post-treatment wound monitoring to provide caregivers with a continuous, quantitative read-out of treatment response and wound healing.
This novel artificial skin device utilizes the cutting-edge and highly advanced microfluidic technology which allows it to not only be portable, but have a significantly smaller size and footprint. Furthermore, this scalable and adjustable skin barrier, would allow a close monitoring of the wound via its transparent nature, and allow strict management of fluids and other modalities that are required for wound maintenance and proper repair.
The Artificial Skin Device consists of two parts, an adhesive sheet of stomahesive to which is fused the microfluidic and transparent artificial Skin. This device once in place, defines a closed chamber over the wound which has several entry and exit ports for supply of the irrigating fluid, cleansing fluid, topical nutritional support fluid and oxygen delivery as well as escape of the fluid and wound exudate respectively. The entry ports accommodate standard i.v. set, while the exit is connected to a drainage bag which may be emptied through a tap. All ports can be closed off when not in use. All inflows are connected to small programmable pumps. Stomahesive is a preparation of gelatin, pectin, sodium carboxymethylcellulose, and polyisobutylene that is non-allergenic and sticks avidly to moist skin as a protective cover and to excoriated skin to promote rapid healing.
The artificial skin device provides a clean and effective method of applying different solutions (irrigation, cleansing and nutritional support) to the wound surface. The features of this artificial skin –the wound irrigation- device to convey the following advantages over conventional dressings:
a) Provides a closed system for wound irrigation.
b) Reduces exposure of the wounds to the atmosphere and eliminates handling of contaminated materials.
c) Prevents maceration of the surrounding skin by soggy dressings or fistula effluent.
d) Eliminates unpleasant smell.
e) Proves cost-effective by saving nursing time spent on change of dressings.
f) Provides a system for the evaluation of topical antiseptics, antibiotics, and other solutions or gases in the treatment of sepsis and promotion of wound healing.
-
Awards
-
 2020 Top 100 Entries
2020 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Mordechai Nosrati
- Type of entry:individual
- Software used for this entry:no
- Patent status:patented