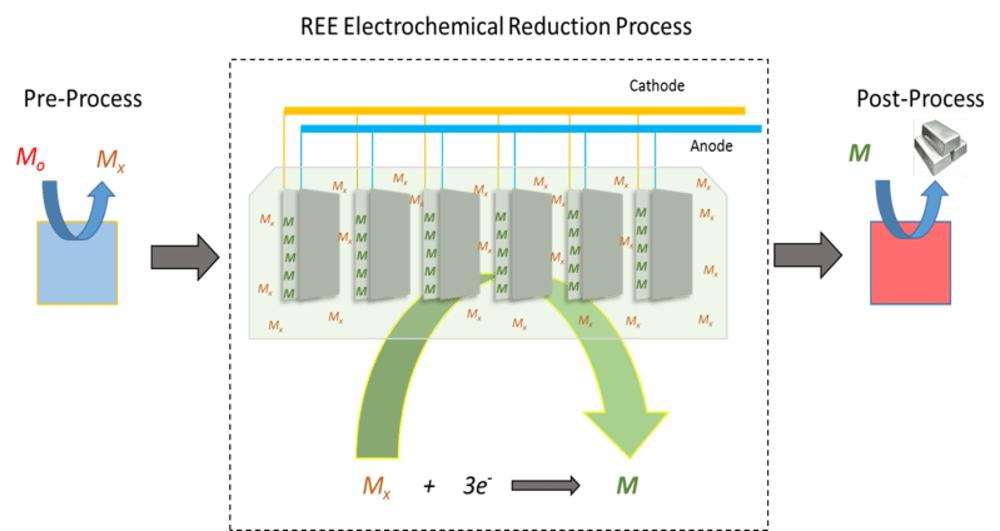
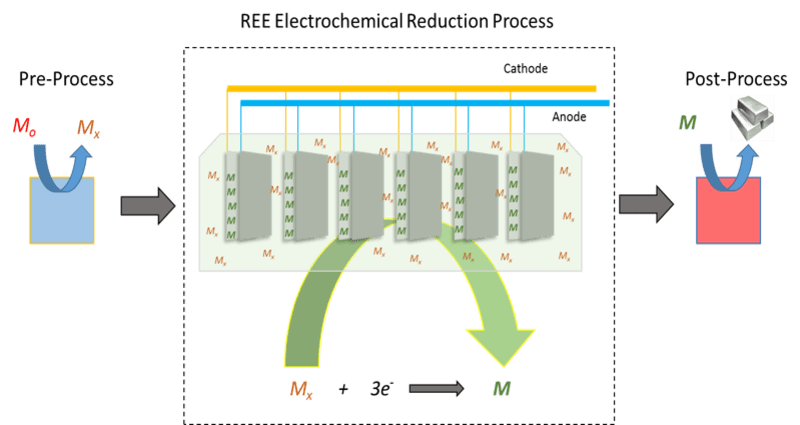
This technology is a new rare earth element (REE) recovery process using a room temperature ionic liquid solution. Current technologies for producing REEs require high temperatures and produce an abundance of toxic fluoride salt waste. These limitations create a need for an environmentally friendly, low temperature technology to produce rare earth metals. This process operates at lower temperatures in an energy efficient and environmentally safe manner.
This technology would be able to separate and purify rare earth metals. The metal is added to the ionic liquid solution as a salt or metal complex. A secondary, tertiary, quaternary, etc. component (gas, liquid, salt, or supercritical fluid) is added to the room temperature ionic liquid solution to reduce the viscosity and enhance solubility and mass transfer. By using a supercritical fluid, the metal complex can be delivered to the ionic liquid solution, diffusion of the metal is enhanced, and electrochemical byproducts can be removed to keep the electrochemical process continuously operating.
Benefits
- Energy efficient
- Environmentally friendly
- Lowers the required process temperature from 900C down to 40 C
Applications
- Rare earth metal production
- Recycling of magnets
- Recovery of rare earth metals from mine tailings
-
Awards
-
 2021 Top 100 Entries
2021 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Andrew Rankin
- Type of entry:teamTeam members:Donna Baek, Robert Fox, Tedd Lister
- Patent status:pending